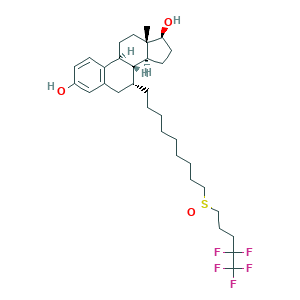
| Synonyms |
Faslodex; Fulvestrant; Fulvestrant (Faslodex); ZD 182780; ZD 9238; ZD-9238; ZM 182780; ZM-182780; (7R,8R,9S,13S,14S,17S)-13-methyl-7-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl]-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol; 129453-61-8; 7alpha-(9-((4,4,5,5,5-Pentafluoropentyl)sulfinyl)nonyl)estra-1,3,5(10)-triene-3,17beta-diol; C32H47F5O3S; CHEBI:31638; CHEMBL1358; DSSTox_CID_2369; DSSTox_GSID_22369; DSSTox_RID_76561; ICI 182,780; ICI-182780; Ici 182780
|
| Cross-matching ID |
- PubChem CID
- 104741
- PubChem SID
-
534986
; 7848224
; 8139859
; 10233578
; 12014659
; 14887338
; 14911923
; 44434356
; 47213214
; 48421875
; 49965830
; 53787559
; 56310723
; 56311014
; 56311884
; 56311948
; 56312955
; 57337809
; 57570890
; 74779769
; 87325152
; 90341164
; 92309278
; 92721810
; 93165800
; 93167154
; 99437215
; 103447197
; 104054514
; 104372812
; 121360934
; 124757068
; 125163872
; 126627510
; 126634728
; 126666851
; 129538438
; 134337631
; 135060458
; 135650370
; 135916581
; 136946608
; 137267475
; 143493372
; 144115998
; 144204490
; 144209802
; 144213958
; 152035088
; 152233184
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0JO7Y
- Formula
- C32H47F5O3S
- Canonical SMILES
- CC12CCC3C(C1CCC2O)C(CC4=C3C=CC(=C4)O)CCCCCCCCCS(=O)CCCC(C(F)(F)F)(F)F
- InChI
- 1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1
- InChIKey
- VWUXBMIQPBEWFH-WCCTWKNTSA-N
|