Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1009) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Medifoxamine
|
|||||
| Synonyms |
Medifoxamina; Medifoxamina [INN-Spanish]; Medifoxamine; Medifoxamine (INN); Medifoxamine [INN:DCF]; Medifoxaminum; Medifoxaminum [INN-Latin]; KWU7C2A1NT; ZX-AT022754; (Dimethylamino)acetaldehyde diphenyl acetal; 32359-34-5; AC1L1V3V; Acetaldehyde, (dimethylamino)-, diphenyl acetal; BRN 2054310; C16H19NO2; CHEBI:135061; CHEMBL85231; CTK4G8876; DTXSID80186078; EINECS 251-011-4; Ethanamine, N,N-dimethyl-2,2-diphenoxy-; N,N-Dimethyl-2,2-diphenoxyethanamine; N,N-Dimethyl-2,2-diphenoxyethylamine; SCHEMBL49319; UNII-KWU7C2A1NT
|
|||||
| Indication | Depression [ICD11: 6A71] | Phase 4 | [1] | |||
| Structure |
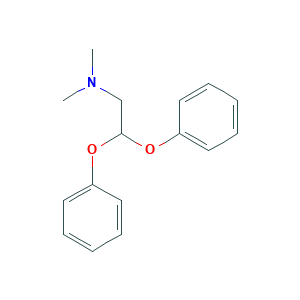 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 257.329 | Topological Polar Surface Area | 21.7 | ||
| Heavy Atom Count | 19 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 3 | |||
| Cross-matching ID | ||||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

