Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1029) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Meprobamate
|
|||||
| Synonyms |
Mepantin; Mepavlon; Mepiosine; Mepranil; Meprindon; Meprobam; Meprobamat; Meproban; Meprocompren; Meprocon; Meprodil; Meproleaf; Meprosan; Meprosin; Meprospan; Meprotabs; Meprotan; Meprotil; Metractyl; Metranquil; Miltamato; Miltown; Neuramate; Tranmep; Amepromat; Anastress; Anathylmon; Anatimon; Andaksin; Ansiatan; Ansiowas; Anxietil; Auxietil; Ayeramate; Biobamat; Biobamate; Calmadin; Calmiren; Carbaxin; Cirponyl; Crestanil; Despasmol; Dicandiol; Equanil; Equatrate; Equilium; Harmonin; Holbamate; Ipsotian; Lepetown; Libiolan; Margonil; meprobamate; Dapaz
|
|||||
| Indication | Anxiety disorder [ICD11: 6B00] | Approved | [1] | |||
| Structure |
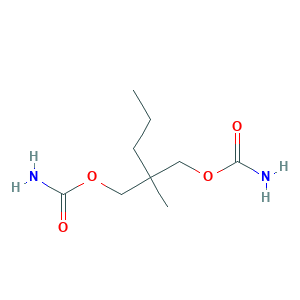 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 218.25 | Topological Polar Surface Area | 105 | ||
| Heavy Atom Count | 15 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 4 | |||
| Cross-matching ID |
|
|||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

