| General Information of Drug (ID:
DR1088) |
| Drug Name |
Midodrine
|
| Prodrug Info |
Midodrine is the prodrug of Desglymidodrine
|
| Synonyms |
Midodrin; Midodrina; Midodrina [INN-Spanish]; Midodrine [INN:BAN]; Midodrinum; Midodrinum [INN-Latin]; ProAmatine; ST-1085; St 1085; midodrine; (RS)-N'-(beta-Hydroxy-2,5-dimethoxy-phenethyl)glycinamid; 1-(2',5'-Dimethoxyphenyl)-2-glycinamidoethanol; 2-Amino-N-(2,5-dimethoxy-beta-hydroxyphenethyl)acetamid; 2-Amino-N-(2,5-dimethoxy-beta-hydroxyphenethylacetamide; 2-amino-N-[2-(2,5-dimethoxyphenyl)-2-hydroxyethyl]acetamide; 42794-76-3; CHEBI:6933; DL-N1-(beta-Hydroxy-2,5-dimethoxyphenethyl)glycinamid; EINECS 255-945-3
|
| Indication |
Orthostatic hypotension
[ICD11: BA21]
|
Approved
|
[1]
|
| Structure |
|
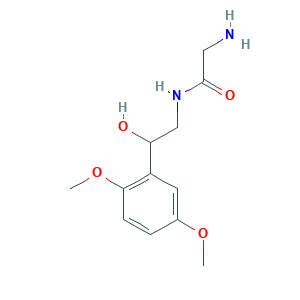 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
254.28 |
Topological Polar Surface Area |
93.8 |
| Heavy Atom Count |
18 |
Rotatable Bond Count |
6 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 4195
- PubChem SID
-
10092
; 7433691
; 8152622
; 11336130
; 11361369
; 11364535
; 11367097
; 11369659
; 11372749
; 11373902
; 11377821
; 11462341
; 11466219
; 11467339
; 11485126
; 11485798
; 11489361
; 11491400
; 11492007
; 11495455
; 14847816
; 29223300
; 46507373
; 47365278
; 47365279
; 47662374
; 47736573
; 47810835
; 48035214
; 48259327
; 48416280
; 49698958
; 50064787
; 50122851
; 50122852
; 50949507
; 56464179
; 57322186
; 85788446
; 96024908
; 104305704
; 124963568
; 125684389
; 128965708
; 134222029
; 134337543
; 135002277
; 137240087
; 137248546
; 142173049
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02XJY
- Formula
- C12H18N2O4
- Canonical SMILES
- COC1=CC(=C(C=C1)OC)C(CNC(=O)CN)O
- InChI
- 1S/C12H18N2O4/c1-17-8-3-4-11(18-2)9(5-8)10(15)7-14-12(16)6-13/h3-5,10,15H,6-7,13H2,1-2H3,(H,14,16)
- InChIKey
- PTKSEFOSCHHMPD-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


