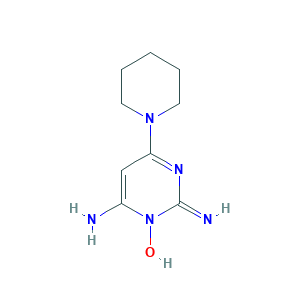
| Synonyms |
Minodyl; Minossidile [Italian]; Minoxidilum; Minoxidilum [INN-Latin]; Minoximen; Normoxidil; Prexidil; Regaine; Rogaine; Theroxidil; Tricoxidil; U-10858; Alopexil; Alostil; Apo-Gain; Avacor and Mintop; Loniten; Lonolox; minoxidil; 2,4-Diamino-6-piperidinopyrimidine 3-oxide; 2,4-Pyrimidinediamine, 6-(1-piperidinyl)-, 3-oxide; 2,6-Diamino-4-(piperidin-1-yl)pyrimidine 1-oxide; 38304-91-5; 6-(1-Piperidinyl)-2,4-pyrimidinediamine 3-oxide; 6-(piperidin-1-yl)pyrimidine-2,4-diamine 3-oxide; CHEBI:6942; MFCD00063409
|
| Cross-matching ID |
- PubChem CID
- 4201
- PubChem SID
-
855572
; 4428325
; 6790804
; 7847484
; 7980000
; 8149432
; 8152627
; 10321504
; 11111457
; 11111458
; 11113580
; 11119965
; 11120453
; 11120941
; 11121450
; 11121930
; 11147048
; 11335335
; 11360574
; 11362519
; 11363863
; 11365081
; 11366425
; 11367643
; 11368987
; 11370309
; 11370310
; 11371637
; 11373244
; 11373724
; 11375805
; 11377149
; 11461546
; 11466048
; 11467168
; 11484742
; 11485716
; 11488869
; 11490325
; 11491878
; 11494783
; 12013813
; 12016118
; 14797675
; 15121148
; 17405347
; 24715035
; 26512261
; 26611827
; 26679549
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Y2CJ
- Formula
- C9H15N5O
- Canonical SMILES
- C1CCN(CC1)C2=NC(=N)N(C(=C2)N)O
- InChI
- 1S/C9H15N5O/c10-7-6-8(12-9(11)14(7)15)13-4-2-1-3-5-13/h6H,1-5,10H2,(H2,11,12)
- InChIKey
- ZIMGGGWCDYVHOY-UHFFFAOYSA-N
|