| General Information of Drug (ID:
DR1210) |
| Drug Name |
Oxaliplatin
|
| Synonyms |
Oxaliplatin (Eloxatin); Oxaliplatin (JAN/USAN/INN); SC-17287; SCHEMBL4859; SW219151-1; 15171-EP2272827A1; 15171-EP2292617A1; 15171-EP2295055A2; Eloxatin (TN); 15171-EP2295416A2; 15171-EP2295427A1; 15171-EP2298748A2; 15171-EP2298764A1; 15171-EP2298765A1; 15171-EP2298768A1; 15171-EP2298778A1; 15171-EP2298780A1; 15171-EP2305642A2; 15171-EP2305671A1; 15171-EP2308855A1; 15171-EP2311453A1; 15171-EP2311825A1; 15171-EP2311829A1; 15171-EP2311840A1; 15171-EP2316832A1; 15171-EP2316833A1; AB01568250_01; AKOS015920125; BR-72813; D01790; O0372; s1224
|
| Indication |
Colorectal cancer
[ICD11: 2B91]
|
Approved
|
[1]
|
| Structure |
|
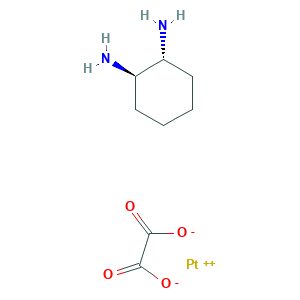 |
|
3D MOL is unavailable
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
397.3 |
Topological Polar Surface Area |
132 |
| Heavy Atom Count |
15 |
Rotatable Bond Count |
0 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
6 |
| Cross-matching ID |
- PubChem CID
- 9887054
- PubChem SID
-
7848852
; 14854529
; 14903423
; 24173438
; 45633994
; 57373686
; 78790585
; 125309438
; 127301221
; 127301222
; 127301223
; 127301224
; 127301225
; 127301226
; 127301227
; 127301228
; 127301229
; 127301230
; 127301231
; 127301232
; 127301233
; 127301234
; 127301235
; 127301236
; 127301237
; 127301238
; 127301239
; 127301240
; 127301241
; 127301242
; 127301243
; 127301244
; 127301245
; 127301246
; 135684119
; 137001870
; 152059544
; 162036207
; 164196639
; 174477519
; 226396770
; 251885690
; 252156659
; 252316548
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Y3ME
- Formula
- C8H14N2O4Pt
- Canonical SMILES
- C1CCC(C(C1)N)N.C(=O)(C(=O)[O-])[O-].[Pt+2]
- InChI
- 1S/C6H14N2.C2H2O4.Pt/c7-5-3-1-2-4-6(5)8;3-1(4)2(5)6;/h5-6H,1-4,7-8H2;(H,3,4)(H,5,6);/q;;+2/p-2/t5-,6-;;/m1../s1
- InChIKey
- ZROHGHOFXNOHSO-BNTLRKBRSA-L
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


