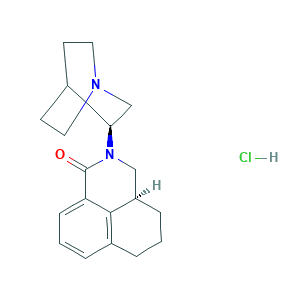
| Synonyms |
Palonosetron (Hydrochloride); Palonosetron hydrochloride; RS 25259-197; RS-25259-197; (3aS)-2-(3S)-1-Azabicyclo[2.2.2]oct-3-yl-2,3,3a,4,5,6-hexahydro-1H-benz[de]isoquinolin-1-one monohydrochloride; (3aS)-2-[(3S)-1-azabicyclo[2.2.2]octan-3-yl]-3a,4,5,6-tetrahydro-3H-benzo[de]isoquinolin-1-one hydrochloride; (S,S)-Palonosetron Hydrochloride; 135729-62-3; 23310D4I19; CAS-135729-62-3; CHEBI:85157; DSSTox_CID_26610; DSSTox_GSID_46610; DSSTox_RID_81765; UNII-1J5X5HPB4C; UNII-23310D4I19; Onicit; PALONOSETRON HCl
|
| Cross-matching ID |
- PubChem CID
- 6918303
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D04FVU
- Formula
- C19H25ClN2O
- Canonical SMILES
- C1CC2CN(C(=O)C3=CC=CC(=C23)C1)C4CN5CCC4CC5.Cl
- InChI
- 1S/C19H24N2O.ClH/c22-19-16-6-2-4-14-3-1-5-15(18(14)16)11-21(19)17-12-20-9-7-13(17)8-10-20;/h2,4,6,13,15,17H,1,3,5,7-12H2;1H/t15-,17-;/m1./s1
- InChIKey
- OLDRWYVIKMSFFB-SSPJITILSA-N
|