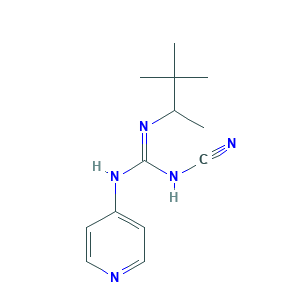
| Synonyms |
Pinacidil anhydrous; Pinacidilum; Pinacidilum [INN-Latin]; S 1230; S-1230; pinacidil; (+-)-Pinacidil; (R,S)-Pinacidil; 1-cyano-2-(3,3-dimethylbutan-2-yl)-3-pyridin-4-ylguanidine; 2-Cyano-3-(4-pyridinyl)-1-(1,2,2-trimethylpropyl)guanidine; P 1134; 60560-33-0; 85371-64-8; CHEMBL1159; EINECS 262-294-9; Guanidine, N-cyano-N'-4-pyridinyl-N''-(1,2,2-trimethylpropyl)-; MLS000069377; N-Cyano-N'-4-pyridinyl-N''-(1,2,2-trimethylpropyl)guanidine; P-154; SMR000058360
|
| Cross-matching ID |
- PubChem CID
- 4826
- PubChem SID
-
4669683
; 8152962
; 10321422
; 11120045
; 11120533
; 11121021
; 11121526
; 11122006
; 11147128
; 11342030
; 11362213
; 11362595
; 11363281
; 11365157
; 11365843
; 11367719
; 11368405
; 11370461
; 11370462
; 11373320
; 11375881
; 11376567
; 11466274
; 11467394
; 11485554
; 11485987
; 11487615
; 11489555
; 11494201
; 11532877
; 14892701
; 15196571
; 17405595
; 24277866
; 26613193
; 26679753
; 26749106
; 26749107
; 26752205
; 26752206
; 29223908
; 47425317
; 47721339
; 47721634
; 47944819
; 47944820
; 48019685
; 48020021
; 48170469
; 48244097
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D02KMO
- Formula
- C13H19N5
- Canonical SMILES
- CC(C(C)(C)C)N=C(NC#N)NC1=CC=NC=C1
- InChI
- 1S/C13H19N5/c1-10(13(2,3)4)17-12(16-9-14)18-11-5-7-15-8-6-11/h5-8,10H,1-4H3,(H2,15,16,17,18)
- InChIKey
- IVVNZDGDKPTYHK-UHFFFAOYSA-N
|