| Synonyms |
Praziquantel (Biltricide); Praziquantelum [INN-Latin]; Pyquiton; praziquantel; Azinox; Biliricide; Biltricide; Cutter Tape Tabs; Droncit; Embay 8440; FSVJFNAIGNNGKK-UHFFFAOYSA-N; 2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4H-pyrazino[2,1-a]isoquinolin-4-one; 2-cyclohexanecarbonyl-1H,2H,3H,4H,6H,7H,11bH-piperazino[2,1-a]isoquinolin-4-one; 4H-Pyrazino[2,1-a]isoquinolin-4-one, 2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-; 55268-74-1; BRN 0761557; CCRIS 4114; Cesol; EINECS 259-559-6; MFCD00058531; MLS000038419; SMR000037139
|
| Cross-matching ID |
- PubChem CID
- 4891
- PubChem SID
-
9571
; 603043
; 855582
; 858012
; 4500852
; 7847537
; 7980375
; 8149489
; 8153009
; 10321427
; 10589244
; 11335305
; 11360544
; 11364013
; 11366575
; 11369137
; 11371799
; 11374102
; 11377299
; 11461516
; 11466288
; 11467408
; 11485031
; 11486035
; 11489104
; 11490366
; 11492297
; 11494933
; 12015327
; 15272465
; 17405467
; 22389787
; 24278635
; 24870350
; 26611882
; 26680161
; 26747203
; 26747204
; 29223969
; 46507082
; 47217033
; 47291354
; 47589213
; 47662536
; 48035386
; 48185224
; 48259483
; 48259484
; 48334763
; 48414364
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0L9ZR
- Formula
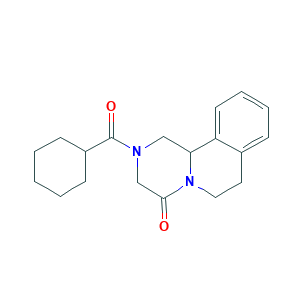
- C19H24N2O2
- Canonical SMILES
- C1CCC(CC1)C(=O)N2CC3C4=CC=CC=C4CCN3C(=O)C2
- InChI
- 1S/C19H24N2O2/c22-18-13-20(19(23)15-7-2-1-3-8-15)12-17-16-9-5-4-6-14(16)10-11-21(17)18/h4-6,9,15,17H,1-3,7-8,10-13H2
- InChIKey
- FSVJFNAIGNNGKK-UHFFFAOYSA-N
|