| General Information of Drug (ID:
DR1377) |
| Drug Name |
CBL-0102
|
| Synonyms |
Acrinamine; Acriquine; Akrichin; Antimalarina; Atebrine; Haffkinine; Italchin; Italchine; Malaricida; Mecryl; Mepacrina [INN-Spanish]; Mepacrine [INN:BAN]; Mepacrinum; Mepacrinum [INN-Latin]; Methoquine; Metochin; Palacrin; Palusan; Pentilen; Quinacrine hydrochloride; Quinacrine, Mepacrine; Quinactine; St 439; acrichine; atabrine; atebrin; mepacrine; quinacrine; 2-Methoxy-6-chloro-9-diethylaminopentylaminoacridine; 3-Chloro-7-methoxy-9-(1-methyl-4-diethylaminobutylamino)acridine; 83-89-6; Erion; HSDB 3253
|
| Indication |
Creutzfeldt-Jakob disease
[ICD11: 8E02]
|
Phase 2
|
[1]
|
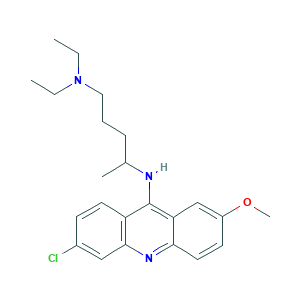
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
400 |
Topological Polar Surface Area |
37.4 |
| Heavy Atom Count |
28 |
Rotatable Bond Count |
9 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 237
- PubChem SID
-
9546
; 598066
; 3138736
; 5198883
; 8150522
; 10589337
; 11335276
; 11360515
; 11363823
; 11364917
; 11366385
; 11367479
; 11368947
; 11370041
; 11371712
; 11373080
; 11374150
; 11375641
; 11377109
; 11378211
; 11405321
; 11461487
; 11466346
; 11467466
; 11484412
; 11486271
; 11488704
; 11490422
; 11492311
; 11494743
; 11533330
; 14781277
; 24262994
; 24438614
; 25820836
; 46507828
; 47365028
; 47440097
; 47736309
; 48034955
; 48034956
; 48110303
; 48110304
; 48184845
; 48334330
; 48415587
; 49698889
; 49864262
; 50111199
; 53790823
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0O2DQ
- Formula
- C23H30ClN3O
- Canonical SMILES
- CCN(CC)CCCC(C)NC1=C2C=C(C=CC2=NC3=C1C=CC(=C3)Cl)OC
- InChI
- 1S/C23H30ClN3O/c1-5-27(6-2)13-7-8-16(3)25-23-19-11-9-17(24)14-22(19)26-21-12-10-18(28-4)15-20(21)23/h9-12,14-16H,5-8,13H2,1-4H3,(H,25,26)
- InChIKey
- GPKJTRJOBQGKQK-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


