| Synonyms |
Raltegravir ( K salt) API; Raltegravir (potassium salt); Raltegravir Potassium Salt; Raltegravir potassium; Raltegravir potassium [USAN:JAN]; Raltegravir(MK-0518); RaltegravirPotassiumSalt; Raltegravir; Raltegravir (MK-0518); MK-0518; MK0518; UNII-22VKV8053U; N-(2-(4-((4-fluorobenzyl)carbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-yl)-5-methyl-1,3,4-oxadiazole-2-carboxamide; MK 0518; 22VKV8053U; NCGC00184997-01; AK326327; DSSTox_CID_28586; DSSTox_RID_82857; DSSTox_GSID_48660; N-(2-(4-(4-Fluorobenzylcarbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-yl)-5-methyl-1,3,4-oxadiazole-2-carboxamide; 518048-05-0; Isentress; UNII-43Y000U234; potassium 4-((4-fluorobenzyl)carbamoyl)-1-methyl-2-(2-(5-methyl-1,3,4-oxadiazole-2-carboxamido)propan-2-yl)-6-oxo-1,6-dihydropyrimidin-5-olate; raltegravir-potassium; 43Y000U234; 871038-72-1; AK326594; C20H20FN6O5.K; D07133; Isentress (TN); MK-518; PubChem22484; Q-201657
|
| Cross-matching ID |
- PubChem CID
- 23668479
- CAS Number
-
- TTD Drug ID
- D0I1FQ
- Formula
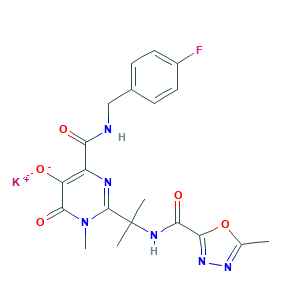
- C20H20FKN6O5
- Canonical SMILES
- CC1=NN=C(O1)C(=O)NC(C)(C)C2=NC(=C(C(=O)N2C)[O-])C(=O)NCC3=CC=C(C=C3)F.[K+]
- InChI
- 1S/C20H21FN6O5.K/c1-10-25-26-17(32-10)16(30)24-20(2,3)19-23-13(14(28)18(31)27(19)4)15(29)22-9-11-5-7-12(21)8-6-11;/h5-8,28H,9H2,1-4H3,(H,22,29)(H,24,30);/q;+1/p-1
- InChIKey
- IFUKBHBISRAZTF-UHFFFAOYSA-M
|