Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1416) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Riboflavin
|
|||||
| Synonyms |
Ribipca; Ribocrisina; Riboderm; Riboflavina; Riboflavina [INN-Spanish]; Riboflavine; Riboflavine [INN-French]; Riboflavinequinone; Riboflavinum; Riboflavinum [INN-Latin]; Ribosyn; Ribotone; Ribovel; Russupteridine Yellow III; Vitaflavine; Vitamin Bi; Aqua-Flave; Beflavin; Beflavine; Dermadram; Fiboflavin; Flavaxin; Flavin BB; Flaxain; Hyflavin; Lactobene; Lactoflavin; Lactoflavine; Vitamin G; Vitasan B2; riboflavin; vitamin B2; (-)-riboflavin; 6,7-Dimethyl-9-D-ribitylisoalloxazine; 7,8-Dimethyl-10-ribitylisoalloxazine; 83-88-5; E101; HYRE
|
|||||
| Indication | Acne vulgaris [ICD11: ED80] | Approved | [1] | |||
| Structure |
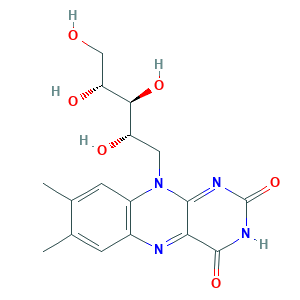 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 376.4 | Topological Polar Surface Area | 155 | ||
| Heavy Atom Count | 27 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 5 | Hydrogen Bond Acceptor Count | 7 | |||
| Cross-matching ID |
|
|||||
| The Predicted Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Predicted Drug Metabolites (PDM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

