| Synonyms |
Taturil; Teriam; Teridin; Tri-Span; Triamizide; Triampur; Triamteren; Triamterenum; Triamteril; Triamteril complex; Triamthiazid; Tricilone; Trispan; Triteren; Triurene; Urocaudal; triamterene; 2,4,7-Triamino-6-phenylpteridine; 396-01-0; Ademin; Ademine; Dinazide; Diucelpin; Diurene; Diutensat; Diuteren; Dyberzide; Dyrenium; Dytenzide; Esiteren; Hidiurese; Hypertorr; Jatropur; Jenateren; Kalspare; Masuharmin; Noridil; Noridyl; Pterofen; Pterophene; Renezide; 6-Phenyl-2,4,7-pteridinetriamine; 6-phenylpteridine-2,4,7-triamine; Diren; Ditak; Dyren; Dytac
|
| Cross-matching ID |
- PubChem CID
- 5546
- PubChem SID
-
118408
; 602599
; 855896
; 933823
; 5660597
; 7847452
; 8036221
; 8149565
; 8153414
; 10321477
; 11111878
; 11111879
; 11335608
; 11360847
; 11363402
; 11365964
; 11368526
; 11372243
; 11373970
; 11376688
; 11452214
; 11461819
; 11466062
; 11467182
; 11484612
; 11485784
; 11488754
; 11491187
; 11492181
; 11494322
; 14774401
; 17389703
; 17405750
; 24277877
; 26611958
; 26679801
; 26747035
; 26751552
; 29224587
; 46507623
; 47291080
; 47588940
; 47662218
; 47662219
; 47810690
; 47885351
; 47885352
; 47959674
; 48035047
; 48334426
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D00NKB
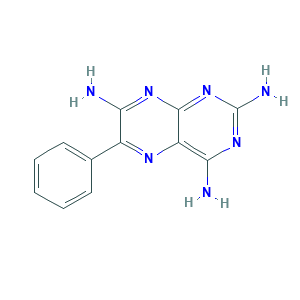
- Formula
- C12H11N7
- Canonical SMILES
- C1=CC=C(C=C1)C2=NC3=C(N=C(N=C3N=C2N)N)N
- InChI
- 1S/C12H11N7/c13-9-7(6-4-2-1-3-5-6)16-8-10(14)18-12(15)19-11(8)17-9/h1-5H,(H6,13,14,15,17,18,19)
- InChIKey
- FNYLWPVRPXGIIP-UHFFFAOYSA-N
|