Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1725) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Zaleplon
|
|||||
| Synonyms |
Zerene; LJC 10846; LJC-10846; S62U433RMH; SKP-1041; Sonata; ZAL 846; ZAL-846; zaleplon; 151319-34-5; 3'-(3-Cyanopyrazolo(1,5-a)pyrimidin-7-yl)-N-ethylacetanilide; 3'-(3-Cyanopyrazolo(1,5-alpha)pyrimidin-7-yl)-N-ethylacetanilide; CHEBI:10102; CL 284,846; CL 284846; CL-284846; DEA No. 2781; L-846; L846; N-(3-(3-Cyanopyrazolo(1,5-a)pyrimidin-7-yl)phenyl)-N-ethylacetamide; N-[3-(3-cyanopyrazolo[1,5-a]pyrimidin-7-yl)phenyl]-N-ethylacetamide; UNII-S62U433RMH; HUNXMJYCHXQEGX-UHFFFAOYSA-N
|
|||||
| Indication | Insomnia [ICD11: 7A00] | Approved | [1] | |||
| Structure |
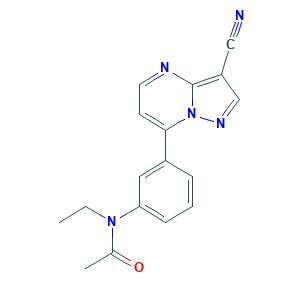 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 305.33 | Topological Polar Surface Area | 74.3 | ||
| Heavy Atom Count | 23 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 0 | Hydrogen Bond Acceptor Count | 4 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

