| General Information of Drug (ID:
DR1755) |
| Drug Name |
Empagliflozin
|
| Synonyms |
Empagliflozin; Empagliflozin (BI 10773); HDC1R2M35U; BI 10773; BI-10773; BI10773; JARDIANCE; (1S)-1,5-anhydro-1-(4-chloro-3-{4-[(3S)-tetrahydrofuran-3-yloxy]benzyl}phenyl)-D-glucitol; (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-(((S)-tetrahydrofuran-3-yl)oxy)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol; 1-chloro-4-(glucopyranos-1-yl)-2-(4-(tetrahydrofuran-3-yloxy)benzyl)benzene; 864070-44-0; CHEBI:82720; UNII-HDC1R2M35U
|
| Indication |
Diabetes mellitus
[ICD11: 5A10]
|
Approved
|
[1]
|
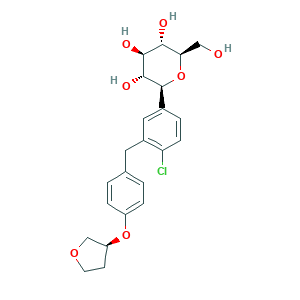
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
450.9 |
Topological Polar Surface Area |
109 |
| Heavy Atom Count |
31 |
Rotatable Bond Count |
6 |
| Hydrogen Bond Donor Count |
4 |
Hydrogen Bond Acceptor Count |
7 |
| Cross-matching ID |
- PubChem CID
- 11949646
- PubChem SID
-
17390987
; 23917761
; 35020536
; 80625411
; 135267035
; 136350032
; 137759455
; 160647541
; 160671533
; 162201844
; 163620871
; 170500874
; 172232552
; 178101464
; 186021010
; 189024748
; 198945623
; 206246269
; 211535427
; 223447440
; 223471392
; 223602217
; 223701070
; 227166637
; 241383513
; 242077478
; 245606277
; 249737155
; 249865857
; 251963060
; 252067199
; 252159682
; 252215347
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D06ALD
- Formula
- C23H27ClO7
- Canonical SMILES
- C1COCC1OC2=CC=C(C=C2)CC3=C(C=CC(=C3)C4C(C(C(C(O4)CO)O)O)O)Cl
- InChI
- 1S/C23H27ClO7/c24-18-6-3-14(23-22(28)21(27)20(26)19(11-25)31-23)10-15(18)9-13-1-4-16(5-2-13)30-17-7-8-29-12-17/h1-6,10,17,19-23,25-28H,7-9,11-12H2/t17-,19+,20+,21-,22+,23-/m0/s1
- InChIKey
- OBWASQILIWPZMG-QZMOQZSNSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


