| General Information of Drug (ID:
DR1763) |
| Drug Name |
Decitabine
|
| Synonyms |
Dacogen; Decitabine; Dezocitidine; 2'-Deoxy-5-azacytidine; 2353-33-5; 4-Amino-1-(2-deoxy-beta-D-erythro-pentofuranosyl)-1,3,5-triazin-2(1H)-one; 4-amino-1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-1,3,5-triazin-2(1H)-one; 5-Aza-2′-Deoxycytidine; 5-Aza-2'-deoxycytidine; 5-Azadeoxycytidine; 5-Deoxy-2′-azacytidine; 5-aza-2-deoxycytidine; 5-aza-CdR; 5-aza-dC; 5A2dc; AzadC; CHEBI:50131; Dac; MLS001332587; NSC 127716; UNII-776B62CQ27
|
| Indication |
Myelodysplastic syndrome
[ICD11: 2A36]
|
Approved
|
[1]
|
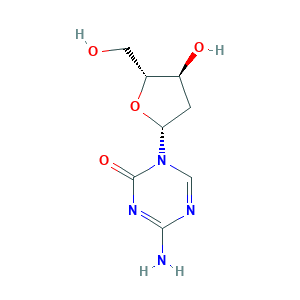
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
228.21 |
Topological Polar Surface Area |
121 |
| Heavy Atom Count |
16 |
Rotatable Bond Count |
2 |
| Hydrogen Bond Donor Count |
3 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 451668
- PubChem SID
-
596457
; 10300747
; 12013916
; 14749266
; 14798099
; 17397761
; 24890797
; 26758275
; 46505657
; 47211573
; 47515034
; 48422583
; 49831907
; 49836662
; 53789316
; 56310803
; 56310829
; 56310991
; 56311079
; 56311138
; 56311371
; 56311372
; 56311435
; 56311466
; 56311472
; 56311487
; 56311581
; 56311739
; 56311918
; 56312280
; 56312302
; 56312939
; 56313048
; 56313052
; 56313366
; 56313487
; 56313832
; 56313833
; 56313869
; 56314010
; 56324652
; 57260149
; 57288417
; 57288447
; 57288645
; 57405281
; 78597402
; 92719107
; 103771112
; 104253166
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0X5XU
- Formula
- C8H12N4O4
- Canonical SMILES
- C1C(C(OC1N2C=NC(=NC2=O)N)CO)O
- InChI
- 1S/C8H12N4O4/c9-7-10-3-12(8(15)11-7)6-1-4(14)5(2-13)16-6/h3-6,13-14H,1-2H2,(H2,9,11,15)/t4-,5+,6+/m0/s1
- InChIKey
- XAUDJQYHKZQPEU-KVQBGUIXSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


