Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1787) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
CBL-954
|
|||||
| Synonyms |
Tretazicar; Tretazicar [INN]; 2,4-Dinitro-5-ethyleneiminobenzamide; 2,4-Dinitroethyleneiminobenzamide; 21919-05-1; 5-(1-Aziridinyl)-2,4-dinitrobenzamide; 5-(AZIRIDIN-1-YL)-2,4-DINITROBENZAMIDE; 5-Aziridino-2,4-dinitrobenzamide; 5-Aziridinyl-2,4-dinitrobenzamide; 5-[aziridin-1-yl]-2,4-dinitrobenzamide; 7865D5D01M; BRN 5825582; Benzamide, 5-(1-aziridinyl)-2,4-dinitro-; Benzamide,5-(1-aziridinyl)-2,4-dinitro-; C9H8N4O5; CB 1954; CB-1954; CB1; CB1954; CCRIS 1631; CHEMBL23330; NSC 115829; SMR000326847; UNII-7865D5D01M
|
|||||
| Indication | Hepatocellular carcinoma [ICD11: 2C12] | Discontinued | [1] | |||
| Structure |
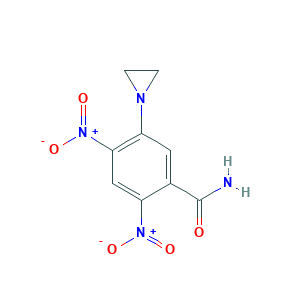 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 252.18 | Topological Polar Surface Area | 138 | ||
| Heavy Atom Count | 18 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 6 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

