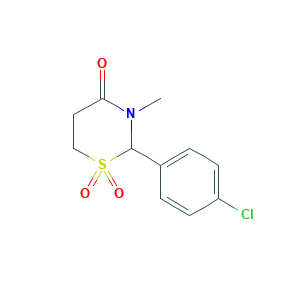
| Synonyms |
Alinam; Banabil-sintyal; Banabin; Banabin-sintyal; Chlormezanone; Banabin-syntyal; Bisina; Chlomedinon; Chlormethazanone; Chlormethazone; Chlormezanon; Chlormezanona; Chlormezanone (JAN/INN); Chlormezanone [BAN:INN:JAN]; Chlormezanone [INN:BAN:JAN]; Chlormezanonum; Chlormezanonum [INN-Latin]; Clorilax; Clormetazanone; Clormetazon; Clormezanona; Clormezanona [INN-Spanish]; Clormezanone; Clormezanone [DCIT]; Dichloromethazanone; Dichloromezanone; Dl-Chlormezanone; Mio-sed; Miorilax; Muskel; Muskel-Trancopal; Myolespen; Phenarol; Rilansyl; Rilaquil; Rilasol; Rilassol; Rillasol; Supotran; Suprotan; TRANCOPAL (TN); Tanafol; Trancopal; Trancopal (TN); Trancote; Tranrilax; Transanate; WIN 4692; (+-)-Chlormezanone; 2-(4-Chlorophenyl)-3-methyl-1,3-thiazinan-4-one 1,1-dioxide; 2-(4-Chlorophenyl)-3-methyl-4-metathiazanone-1,1-dioxide; 2-(4-Chlorophenyl)tetrahydro-3-methyl-4H,1,3-thiazin-4-one 1,1-Dioxide; 2-(4-Chlorophenyl)tetrahydro-3-methyl-4H-1,3-thiazin-4-one 1,1-dioxide; 2-(4-Chlorphenyl)-3-methyl-4-metathiazanon-1,1-dioxid; 2-(4-chlorophenyl)-3-methyl-1,1-dioxo-1,3-thiazinan-4-one; 2-(p-Chlorophenyl)tetrahydro-3-methyl-4H-1,3-thiazin-4-one 1,1-dioxide; 2-(p-Chlorphenyl)-3-methyl-1,3-perhydrothiazin-4-on-1,1-dioxide; 2-(para-Chlorophenyl)tetrahydro-3-methyl-4H-1,3-thiazin-4-one, 1,1-dioxide; 4H-1,3-Thiazin-4-one, 2-(4-chlorophenyl)tetrahydro-3-methyl-, 1,1-dioxide; 4H-1,3-Thiazin-4-one, 2-(4-chlorophenyl)tetrahydro-3-methyl-1,1-dioxide; 4H-1,3-Thiazin-4-one, 2-(p-chlorophenyl)tetrahydro-3-methyl-, 1,1,-dioxide; 4H-1,3-Thiazin-4-one, 2-(p-chlorophenyl)tetrahydro-3-methyl-, 1,1-dioxide; 4H-1,3-Thiazin-4-one, 2-(p-chlorophenyl)tetrahydro-3-methyl-1,1-dioxide; 4H-1,3-Thiazin-4-one, tetrahydro-2-(p-chlorophenyl)-3-methyl-, 1,1-dioxide; C-192; Lobak; Rexan; Rilax
|