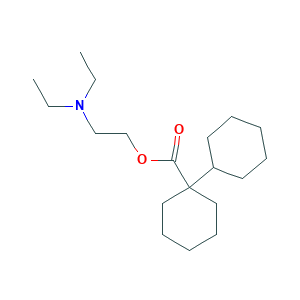
| Synonyms |
Dicicloverina; Dicicloverina [INN-Spanish]; Dicyclomine Hcl; Dicycloverin; Dicycloverin hydrochloride; Dicycloverine; Dicycloverine (INN); Dicycloverine [INN:BAN]; Dicycloverinum; Dicycloverinum [INN-Latin]; Dicymine; Dicymine (TN); Dilomine (TN); Diocyl; Diocyl hydrochloride; Dyspas; Formulex; Formulex (TN); J.L. 998; Kolantyl hydrochloride; Atumin; Bentomine; Bentyl (TN); Bentyl hydrochloride; Bentylol; Bentylol (TN); Bentylol hydrochloride; Di-Spaz (TN); Di-syntramine; Dibent (TN); Lomine (TN); Mamiesan; Merbentyl; Merbentyl (TN); Oxityl-P; Procyclomin; Sawamin; Spasmoban; Wyovin; Wyovin hydrochloride; (1,1'-Bicyclohexyl)-1-carboxylic acid, 2-(diethylamino)ethyl ester; (BICYCLOHEXYL)-1-CARBOXYLIC ACID, 2-(DIETHYLAMINO)ETHYL ESTER, HYDROCHLORIDE; (Bicyclohexyl)-1-carboxylic acid, 2-(diethylamino)ethyl ester; 1-Cyclohexylcyclohexanecarboxylic acid 2-(diethylamino)ethyl ester; 2-(diethylamino)ethyl 1,1'-bi(cyclohexyl)-1-carboxylate; 2-(diethylamino)ethyl 1-cyclohexylcyclohexane-1-carboxylate; 2-diethylaminoethyl 1-cyclohexylcyclohexane-1-carboxylate; 2-diethylaminoethyl 1-cyclohexylcyclohexane-1-carboxylate hydrochloride; Beta-Diethylaminoethyl 1-cyclohexylcyclohexanecarboxylate hydrochloride; Beta-Diethylaminoethyl-1-cyclohexylhexahydrobenzoate hydrochloride; Bicyclohexyl-1-carbonsaeure-2'diethylaminoethylester; Byclomine (TN); Cyclohexanecarboxylic acid, 1-cyclohexyl-, 2-(diethylamino)ethyl ester; Diethylaminocarbethoxybicyclohexyl hydrochloride; GU8471000; [1,1'-Bicyclohexyl]-1-carboxylic acid, 2-(diethylamino)ethyl ester; [Bicyclohexyl]-1-carboxylic acid, 2-(diethylamino)ethyl ester hydrochloride
|