| Synonyms |
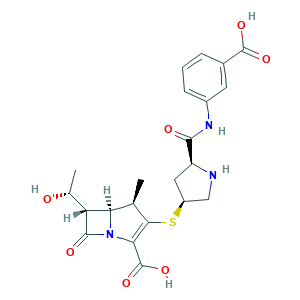
Ertapenem (INN); Ertapenem [INN]; Invanz (TN); (1R,5S,6S,8R,2'S,4'S)-2-(2-(3-carboxyphenylcarbamoyl)pyrrolidin-4-ylthio)-6-(1-hydroxyethyl)-1-methylcarbapenem-3-carboxylic acid; (4R,5S,6S)-3-((3S,5S)-5-((3-carboxyphenyl)carbamoyl)pyrrolidin-3-ylthio)-6-((R)-1-hydroxyethyl)-4-methyl-7-oxo-1-aza-bicyclo[3.2.0]hept-2-ene-2-carboxylic acid; (4R,5S,6S)-3-({(3S,5S)-5-[(3-carboxyphenyl)carbamoyl]pyrrolidin-3-yl}sulfanyl)-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid; (4R,5S,6S)-3-[(3S,5S)-5-[(3-carboxyphenyl)carbamoyl]pyrrolidin-3-yl]sulfanyl-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid
|
| Cross-matching ID |
- PubChem CID
- 150610
- PubChem SID
-
7979182
; 10250773
; 14761086
; 14785384
; 46227255
; 46506508
; 50070556
; 50071303
; 50249601
; 57347055
; 85595963
; 92714224
; 96024604
; 99450921
; 103447288
; 104428898
; 126629618
; 126655559
; 126663131
; 134337727
; 135106916
; 137003431
; 139445158
; 152034496
; 160963651
; 162174325
; 163412145
; 175265685
; 179296050
; 184527505
; 196105532
; 223556604
; 223660209
; 224339593
; 226420912
; 248087834
; 249865868
; 251916745
; 251917984
; 252075643
; 252451452
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Q1MS
- Formula
- C22H25N3O7S
- Canonical SMILES
- CC1C2C(C(=O)N2C(=C1SC3CC(NC3)C(=O)NC4=CC=CC(=C4)C(=O)O)C(=O)O)C(C)O
- InChI
- 1S/C22H25N3O7S/c1-9-16-15(10(2)26)20(28)25(16)17(22(31)32)18(9)33-13-7-14(23-8-13)19(27)24-12-5-3-4-11(6-12)21(29)30/h3-6,9-10,13-16,23,26H,7-8H2,1-2H3,(H,24,27)(H,29,30)(H,31,32)/t9-,10-,13+,14+,15-,16-/m1/s1
- InChIKey
- JUZNIMUFDBIJCM-ANEDZVCMSA-N
|