| Synonyms |
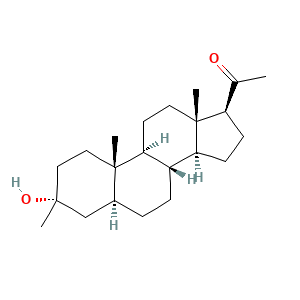
Ganaxolone [USAN]; CCD 1042; CCD-1042; Ganaxolone (USAN/INN); (3alpha,5alpha)-3-Hydroxy-3-methylpregnan-20-one; 1-[(3R,5S,10S,13S,17S)-3-hydroxy-3,10,13-trimethyl-1,2,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]ethanone; 1-[(3R,5S,8R,9S,10S,13S,14S,17S)-3-hydroxy-3,10,13-trimethyl-1,2,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]ethanone; 3alpha-Hydroxy-3-methyl-5alpha-pregnan-20-one; 3alpha-Hydroxy-3beta-methyl-5alpha-pregnan-20-one
|
| Cross-matching ID |
- PubChem CID
- 6918305
- CAS Number
-
- TTD Drug ID
- D0X0CH
- Formula
- C22H36O2
- Canonical SMILES
- CC(=O)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CC[C@@H]4[C@@]3(CC[C@@](C4)(C)O)C)C
- InChI
- InChI=1S/C22H36O2/c1-14(23)17-7-8-18-16-6-5-15-13-20(2,24)11-12-21(15,3)19(16)9-10-22(17,18)4/h15-19,24H,5-13H2,1-4H3/t15-,16-,17+,18-,19-,20+,21-,22+/m0/s1
- InChIKey
- PGTVWKLGGCQMBR-FLBATMFCSA-N
|