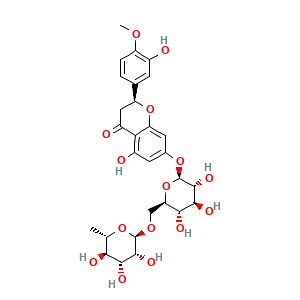
| Synonyms |
hesperidin; 520-26-3; Cirantin; Hesperidoside; Hesper bitabs; Hesperetin-rutinosid; Hesperidine; UNII-E750O06Y6O; CCRIS 3940; (S)-(-)-Hesperidin; EINECS 208-288-1; NSC 44184; BRN 0075140; Hesperidin, (2S)-; Hesperetin 7-rutinoside; MLS001304066; (2S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxy-2,3-dihydrochromen-4-one; CHEBI:28775; USAF CF-3; Hesperidin, (S)-(-)-; Hesperitin-7-rhamnoglucoside; E750O06Y6O; C28H34O15; HESPERIDINE
|
| Cross-matching ID |
- PubChem CID
- 10621
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0I9HF
- Formula
- C28H34O15
- Canonical SMILES
- C[C@H]1[C@@H]([C@H]([C@H]([C@@H](O1)OC[C@@H]2[C@H]([C@@H]([C@H]([C@@H](O2)OC3=CC(=C4C(=O)C[C@H](OC4=C3)C5=CC(=C(C=C5)OC)O)O)O)O)O)O)O)O
- InChI
- InChI=1S/C28H34O15/c1-10-21(32)23(34)25(36)27(40-10)39-9-19-22(33)24(35)26(37)28(43-19)41-12-6-14(30)20-15(31)8-17(42-18(20)7-12)11-3-4-16(38-2)13(29)5-11/h3-7,10,17,19,21-30,32-37H,8-9H2,1-2H3/t10-,17-,19+,21-,22+,23+,24-,25+,26+,27+,28+/m0/s1
- InChIKey
- QUQPHWDTPGMPEX-QJBIFVCTSA-N
|