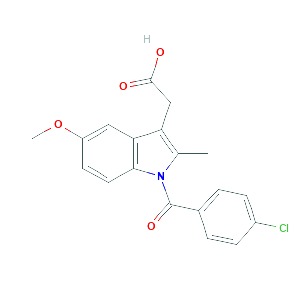
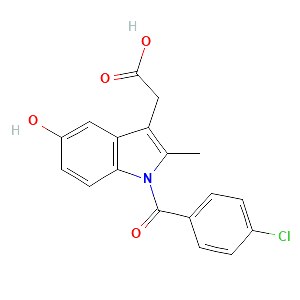
| References |
| 1 |
Comparative in vitro metabolism of phospho-tyrosol-indomethacin by mice, rats and humans Biochem Pharmacol. 2013 Apr 15;85(8):1195-202. doi: 10.1016/j.bcp.2013.01.031.
|
| 2 |
Contribution of UDP-glucuronosyltransferases 1A9 and 2B7 to the glucuronidation of indomethacin in the human liver. Eur J Clin Pharmacol. 2007 Mar;63(3):289-96.
|
| 3 |
Herb-drug interaction in the protective effect of Alpinia officinarum against gastric injury induced by indomethacin based on pharmacokinetic, tissue distribution and excretion studies in rats. J Pharm Anal. 2021 Apr;11(2):200-209. doi: 10.1016/j.jpha.2020.05.009.
|
| 4 |
[Comparative metabolic studies on [14C]-labelled acemetacin and indometacin in rats (author's transl)] Arzneimittelforschung. 1980;30(8A):1384-91.
|