| General Information of Drug (ID:
DR0035) |
| Drug Name |
Acemetacin
|
| Prodrug Info |
Acemetacin is the prodrug of Indomethacin
|
| Synonyms |
Acemetacina; Acemetacina [INN-Spanish]; Acemetacine; Acemetacine [INN-French]; Acemetacinum; Acemetacinum [INN-Latin]; Acemix; Aximeixin; Emflex; Rantudil; Rheumibis; TVX 3322; indometacin carboxymethyl ester; indometacin glycolic ester; indomethacin carboxymethyl ester; indomethacin glycolic ester; 1H-Indole-3-acetic acid, 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-, carboxymethyl ester; 53164-05-9; BRN 0501672; Bay f 4975; C21H18ClNO6; CHEBI:31162; CHEMBL189171; EINECS 258-403-4; K 708; K-708; UNII-5V141XK28X; acemetacin
|
| Indication |
Osteoarthritis
[ICD11: FA00]
|
Phase 4
|
[1]
|
| Structure |
|
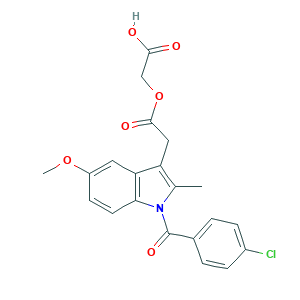 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
415.8 |
Topological Polar Surface Area |
94.8 |
| Heavy Atom Count |
29 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
6 |
| Cross-matching ID |
- PubChem CID
- 1981
- ChEBI ID
-
- CAS Number
-
- Formula
- C21H18ClNO6
- Canonical SMILES
- CC1=C(C2=C(N1C(=O)C3=CC=C(C=C3)Cl)C=CC(=C2)OC)CC(=O)OCC(=O)O
- InChI
- 1S/C21H18ClNO6/c1-12-16(10-20(26)29-11-19(24)25)17-9-15(28-2)7-8-18(17)23(12)21(27)13-3-5-14(22)6-4-13/h3-9H,10-11H2,1-2H3,(H,24,25)
- InChIKey
- FSQKKOOTNAMONP-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


