| General Information of This Metabolic Reaction (MR) (ID:
MR002495) |
| Formula |
|
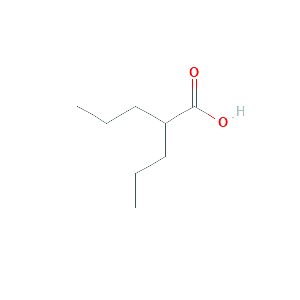
| Reactant |
Valproic acid |
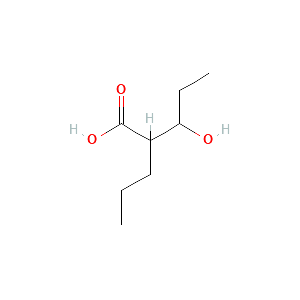
Product |
3-hydroxyvalproic acid |
|
Reactant Info
|
Product Info
|
|
Metabolic Enzyme
|
Cytochrome P450 3A5 (CYP3A5)
|
DME Info
|
|
Cytochrome P450 2A6 (CYP2A6)
|
DME Info
|
|
|
|
|
|
|
|
| Other MR(s) Related to The Reactant of This MR |
|
Other MR(s) That Metabolize The Reactant of This MR
|
|
|
| Other MR(s) Related to The Product of This MR |
|
Other MR(s) That Produce The Product of This MR
|
|
|
|
Other MR(s) That Metabolize The Produtc of This MR
|
|
|
| References |
| 1 |
Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675.
|
| 2 |
PharmGKB: the Pharmacogenomics Knowledge Base Methods Mol Biol. 2013;1015:311-20. doi: 10.1007/978-1-62703-435-7_20.
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.