Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1667) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Valproic acid
|
|||||
| Synonyms |
Valproate; Valproinsaeure; Vupral; Acide valproique; Acido valproico; Acidum valproicum; Avugane; Baceca; Convulex; Depakene; Depakin; Depakine; Deproic; Di-n-propylacetic acid; Di-n-propylessigsaure; Dipropylacetate; Dipropylacetic acid; Ergenyl; Kyselina 2-propylvalerova; Mylproin; Myproic Acid; Pentanoic acid, 2-propyl-; Propylvaleric acid; Stavzor; VALPROIC ACID; n-Dipropylacetic acid; 2-Propylpentanoic acid; 2-Propylvaleric acid; 2-n-Propyl-n-valeric acid; 4-Heptanecarboxylic acid; 99-66-1; Abbott 44090; Acetic acid, dipropyl-; n-DPA
|
|||||
| Indication | Epilepsy [ICD11: 8A60] | Approved | [1] | |||
| Structure |
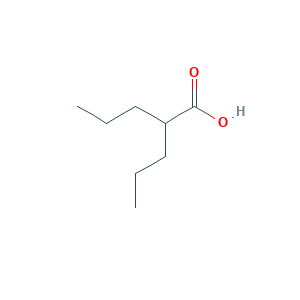 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 144.21 | Topological Polar Surface Area | 37.3 | ||
| Heavy Atom Count | 10 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 2 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | Valproic Acid was approved by FDA. The 2020 official website of the U.S. Food and Drug Administration. | |||||
| 2 | Psychotropic drug interactions with valproate. Clin Neuropharmacol. 2005 Mar-Apr;28(2):96-101. | |||||
| 3 | Insights into CYP2B6-mediated drug-drug interactions. Acta Pharm Sin B. 2016 Sep;6(5):413-425. | |||||
| 4 | A mechanistic approach to antiepileptic drug interactions. Ann Pharmacother. 1998 May;32(5):554-63. | |||||
| 5 | Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675. | |||||
| 6 | Effect of aging on glucuronidation of valproic acid in human liver microsomes and the role of UDP-glucuronosyltransferase UGT1A4, UGT1A8, and UGT1A10. Drug Metab Dispos. 2009 Jan;37(1):229-36. | |||||
| 7 | UDP glucuronosyltransferase (UGT) 1A6 pharmacogenetics: II. Functional impact of the three most common nonsynonymous UGT1A6 polymorphisms (S7A, T181A, and R184S). J Pharmacol Exp Ther. 2005 Jun;313(3):1340-6. | |||||
| 8 | Pharmacokinetic and pharmacodynamic interaction of lorazepam and valproic acid in relation to UGT2B7 genetic polymorphism in healthy subjects. Clin Pharmacol Ther. 2008 Apr;83(4):595-600. | |||||
| 9 | PharmGKB: the Pharmacogenomics Knowledge Base Methods Mol Biol. 2013;1015:311-20. doi: 10.1007/978-1-62703-435-7_20. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

