| References |
| 1 |
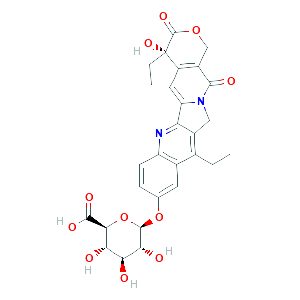
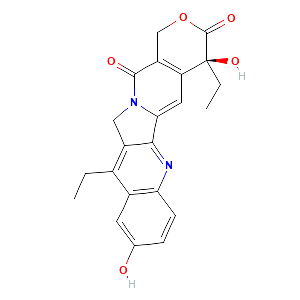
Pharmacokinetics, metabolism, and excretion of irinotecan (CPT-11) following I.V. infusion of [(14)C]CPT-11 in cancer patients Drug Metab Dispos. 2000 Apr;28(4):423-33.
|
| 2 |
Opioid-induced microbial dysbiosis disrupts irinotecan (CPT-11) metabolism and increases gastrointestinal toxicity in a murine model. Br J Pharmacol. 2023 May;180(10):1362-1378. doi: 10.1111/bph.16020.
|
| 3 |
UHPLC-MS/MS method for the quantification of ultra-traces of irinotecan and its metabolites in red blood cells and plasma to detect caregivers' contamination. Drug Test Anal. 2023 Jun 28. doi: 10.1002/dta.3539.
|
| 4 |
Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes
|
| 5 |
Integrated population pharmacokinetics of etirinotecan pegol and its four metabolites in cancer patients with solid tumors
|
| 6 |
Clinical and pharmacologic study of the novel prodrug delimotecan (MEN 4901/T-0128) in patients with solid tumors
|