| General Information of Drug (ID:
DR0053) |
| Drug Name |
Agomelatine
|
| Synonyms |
Agomelatine; Agomelatine [INN]; Melitor; S 20098; S-20098; S20098; Thymanax; Valdoxan; YJYPHIXNFHFHND-UHFFFAOYSA-N; 137R1N49AD; 138112-76-2; AGO-178; Acetamide, N-[2-(7-methoxy-1-naphthalenyl)ethyl]-; C15H17NO2; CHEMBL10878; N-(2-(7-Methoxy-1-naphthalenyl)ethyl)acetamide; N-(2-(7-Methoxynaphth-1-yl)ethyl)acetamide; N-(2-(7-Methoxynaphthalen-1-yl)ethyl)acetamide; N-(2-(7-methoxy-1-naphthyl)ethyl)acetamide; N-[2-(7-Methoxy-1-naphthalenyl)ethyl]acetamide; N-[2-(7-methoxynaphthalen-1-yl)ethyl]acetamide; UNII-137R1N49AD
|
| Indication |
Depression
[ICD11: 6A71]
|
Phase 4
|
[1]
|
| Structure |
|
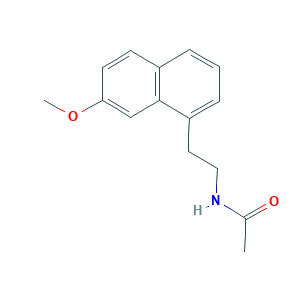 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
243.3 |
Topological Polar Surface Area |
38.3 |
| Heavy Atom Count |
18 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
2 |
| Cross-matching ID |
- PubChem CID
- 82148
- PubChem SID
-
7980554
; 10219207
; 12014766
; 15121987
; 17396749
; 43137308
; 47805575
; 48029689
; 48253997
; 48329090
; 49859801
; 50597493
; 53789947
; 57333351
; 58107135
; 85175740
; 85209276
; 92720043
; 99437046
; 103171284
; 103943227
; 104379867
; 109692881
; 123121579
; 124757110
; 125163914
; 125333716
; 126620366
; 126648120
; 126667094
; 126728359
; 129806556
; 131480735
; 134338989
; 135037533
; 135588690
; 135649906
; 136340261
; 136367368
; 136368118
; 136991142
; 137228670
; 137232583
; 142374863
; 144115558
; 144160950
; 152225873
; 152258747
; 152344557
; 160647592
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0Y8UB
- Formula
- C15H17NO2
- Canonical SMILES
- CC(=O)NCCC1=CC=CC2=C1C=C(C=C2)OC
- InChI
- 1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17)
- InChIKey
- YJYPHIXNFHFHND-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


