| Synonyms |
Alectinib (Hydrochloride); Alectinib Hydrochloride; Alectinib hydrochloride (JAN); Alecensa (TN); CH5424802 HCl; CH5424802 Hydrochloride; P9YY73LO6J; SCHEMBL14991271; UNII-P9YY73LO6J; alectinib HCl; 1256589-74-8; 5H-Benzo[b]carbazole-3-carbonitrile, 9-ethyl-6,11-dihydro-6,6-dimethyl-8-[4-(4-morpholinyl)-1-piperidinyl]-11-oxo-, hydrochloride (1:1); 9-ethyl-6,6-dimethyl-8-[4-(morpholin-4-yl)piperidin-1-yl]-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile hydrochloride; AF-802 hydrochloride
|
| Cross-matching ID |
- PubChem CID
- 53239799
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0U3SY
- Formula
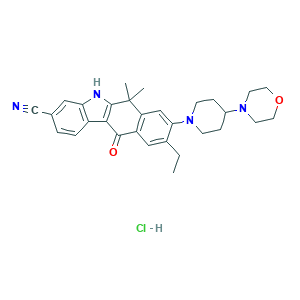
- C30H35ClN4O2
- Canonical SMILES
- CCC1=CC2=C(C=C1N3CCC(CC3)N4CCOCC4)C(C5=C(C2=O)C6=C(N5)C=C(C=C6)C#N)(C)C.Cl
- InChI
- 1S/C30H34N4O2.ClH/c1-4-20-16-23-24(17-26(20)34-9-7-21(8-10-34)33-11-13-36-14-12-33)30(2,3)29-27(28(23)35)22-6-5-19(18-31)15-25(22)32-29;/h5-6,15-17,21,32H,4,7-14H2,1-3H3;1H
- InChIKey
- GYABBVHSRIHYJR-UHFFFAOYSA-N
|