| Cross-matching ID |
- PubChem CID
- 65016
- PubChem SID
-
10286
; 601727
; 628182
; 822019
; 3727052
; 3727059
; 7847958
; 7885327
; 7978703
; 8030478
; 8189448
; 11528777
; 12014852
; 14835820
; 14884574
; 26697364
; 29215412
; 43121862
; 46392146
; 46393211
; 46507537
; 53790789
; 57315242
; 71821412
; 74965413
; 85177028
; 85177034
; 85177055
; 92309266
; 93166556
; 97857368
; 99239834
; 99239838
; 99239842
; 99239846
; 99239847
; 99239854
; 99239856
; 103179760
; 104178998
; 104253275
; 104332645
; 104829267
; 117549941
; 118048728
; 125267472
; 125310993
; 127310203
; 127310204
; 127310205
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0A0OO
- Formula
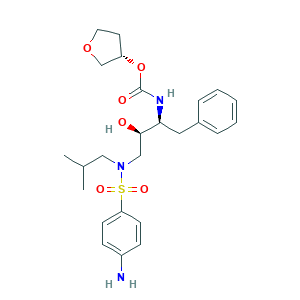
- C25H35N3O6S
- Canonical SMILES
- CC(C)CN(CC(C(CC1=CC=CC=C1)NC(=O)OC2CCOC2)O)S(=O)(=O)C3=CC=C(C=C3)N
- InChI
- 1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1
- InChIKey
- YMARZQAQMVYCKC-OEMFJLHTSA-N
|