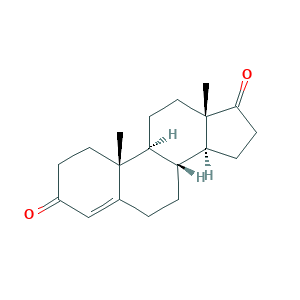
| Synonyms |
Androstendione; Androstenedione [JAN]; Androtex; Fecundin; SKF 2170; androstenedione; d4-androstenedione; delta(sup 4)-Androstene-3,17-dione; delta-(sup4)-Androsten-3,17-dione; delta-4-Androsten-3,17-dione; delta-4-Androstene-3,17-dione; delta-4-Androstenedione; 17-Ketotestosterone; 3,17-Dioxoandrost-4-ene; 4-ANDROSTENE-3-17-DIONE; 4-Androsten-3,17-dione; 4-Androstene-3,17-dione; 4-Androstenedione; Androst-4-ene-3,17-dione; CHEBI:16422; EINECS 200-554-5; HSDB 7335; MLS000028510; NSC 9563; UNII-409J2J96VR
|
| Cross-matching ID |
- PubChem CID
- 6128
- PubChem SID
-
3575
; 75016
; 598429
; 831360
; 841441
; 855972
; 3162127
; 7847119
; 7885977
; 8145630
; 8153839
; 14775551
; 15962866
; 17389251
; 17389252
; 17404677
; 22400337
; 24278247
; 24702267
; 24869681
; 25621509
; 26751578
; 29215020
; 29225131
; 46508011
; 47736685
; 48035332
; 48422859
; 48424700
; 48424701
; 49856256
; 50104208
; 50362263
; 53777220
; 53790644
; 56354941
; 56354943
; 56435876
; 56436206
; 56436633
; 57323206
; 57391483
; 57650669
; 71850513
; 85148334
; 85267761
; 85279313
; 92297726
; 92303593
; 92710448
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0M8RO
- Formula
- C19H26O2
- Canonical SMILES
- CC12CCC(=O)C=C1CCC3C2CCC4(C3CCC4=O)C
- InChI
- 1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-16H,3-10H2,1-2H3/t14-,15-,16-,18-,19-/m0/s1
- InChIKey
- AEMFNILZOJDQLW-QAGGRKNESA-N
|