| General Information of Drug (ID:
DR0236) |
| Drug Name |
Bromperidol
|
| Synonyms |
Bromoperidol; Bromperidolum [INN-Latin]; Impromen; Azurene; LYH6F7I22E; R 11333; RKLNONIVDFXQRX-UHFFFAOYSA-N; Tesoprel; bromperidol; 10457-90-6; 4-(4-(4-Bromophenyl)-4-hydroxy-1-piperidinyl)-1-(4-fluorophenyl)-1-butanone; 4-(4-(4-Bromophenyl)-4-hydroxypiperidino)-4'-fluorobutyrophenone; 4-(4-(4-bromophenyl)-4-hydroxypiperidin-1-yl)-1-(4-fluorophenyl)butan-1-one; 4-(4-(p-Bromophenyl)-4-hydroxypiperidino)-4'-fluorobutyrophenone; BRN 1552256; CAS-10457-90-6; CC 2489; EINECS 233-943-3; NCGC00016692-01; UNII-LYH6F7I22E
|
| Indication |
Schizophrenia
[ICD11: 6A20]
|
Phase 4
|
[1]
|
| Structure |
|
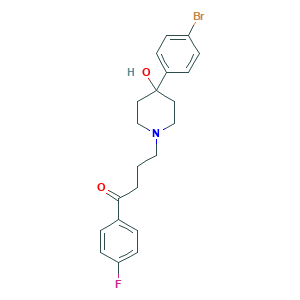 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
420.3 |
Topological Polar Surface Area |
40.5 |
| Heavy Atom Count |
26 |
Rotatable Bond Count |
6 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 2448
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D00SHQ
- Formula
- C21H23BrFNO2
- Canonical SMILES
- C1CN(CCC1(C2=CC=C(C=C2)Br)O)CCCC(=O)C3=CC=C(C=C3)F
- InChI
- 1S/C21H23BrFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2
- InChIKey
- RKLNONIVDFXQRX-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


