Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR0564) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
Eletriptan hydrobromide
|
|||||
| Synonyms |
Eletriptan (hydrobromide); Eletriptan HBr; Eletriptan Hydrobromide [USAN]; Eletriptan hydrobromide; Eletriptan; Eletriptan [INN:BAN]; NCGC00181130-01; Relpax; UK 116044; UK-116044; UK-116044-04; UK-116044-04 [As Hydrobromide); UNII-22QOO9B8KI; (R)-3-((1-methylpyrrolidin-2-yl)methyl)-5-(2-(phenylsulfonyl)ethyl)-1H-indole; 143322-58-1; 22QOO9B8KI; 3-(((R)-1-Methyl-2-pyrrolidinyl)methyl)-5-(2-(phenylsulfonyl)ethyl)indole; 5-[2-(benzenesulfonyl)ethyl]-3-[[(2R)-1-methylpyrrolidin-2-yl]methyl]-1H-indole; 5-[2-(benzenesulfonyl)ethyl]-3-{[(2R)-1-methylpyrrolidin-2-yl]methyl}-1H-indole; CHEBI:50922; DSSTox_RID_81968; M41W832TA3; UK 116044-04; UNII-M41W832TA3; Eletriptan monohydrobromide; (R)-3-((1-Methyl-2-pyrrolidinyl)methyl)-5-(2-(phenylsulfonyl)ethyl)-1H-indole monohydrobromide; (R)-3-((1-Methylpyrrolidin-2-yl)methyl)-5-(2-(phenylsulfonyl)ethyl)-1H-indole hydrobromide; 177834-92-3; 3-[[(2R)-1-Methyl-2-pyrrolidinyl]methyl]-5-[2-(phenylsulfonyl)ethyl]-1H-indole Hydrobromide; CHEBI:61176
|
|||||
| Indication | Migraine [ICD11: 8A80] | Approved | [1] | |||
| Structure |
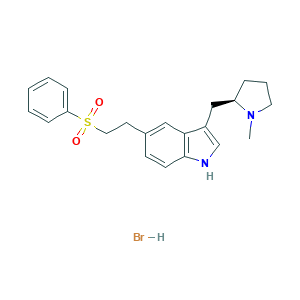 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 463.4 | Topological Polar Surface Area | 61.6 | ||
| Heavy Atom Count | 28 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

