| Synonyms |
Conmana; Icotinib; Icotinib(Lcotinib); Lcotinib; QQLKULDARVNMAL-UHFFFAOYSA-N; SCHEMBL5843603; ZINC43207566; icotinib,BPI-2009H; 2468AH; 610798-31-7; 9G6U5L461Q; BCP21716; BDBM50391089; BPI 2009; BPI-2009; BPI-2009H; BPI2009; CHEMBL2087361; DTXSID20209952; EX-A306; GTPL7641; HMS3651F12; HY-15164A; MFCD22124501; N-(3-ethynylphenyl)-7,8,10,11,13,14-hexahydro-[1,4,7,10]tetraoxacyclododecino[2,3-g]quinazolin-4-amine; N-(3-ethynylphenyl)-7H,8H,10H,11H,13H,14H-1,4,7,10-tetraoxacyclododeca[2,3-g]quinazolin-4-amine; UNII-9G6U5L461Q; s2922
|
| Cross-matching ID |
- PubChem CID
- 22024915
- PubChem SID
-
37614496
; 55538028
; 85182161
; 136920399
; 139213683
; 160707851
; 160870172
; 162009854
; 162108694
; 184815784
; 198986148
; 210275187
; 210280827
; 223365976
; 223856036
; 226087987
; 231627548
; 242060251
; 247237456
; 252472035
- CAS Number
-
- TTD Drug ID
- D06WRJ
- Formula
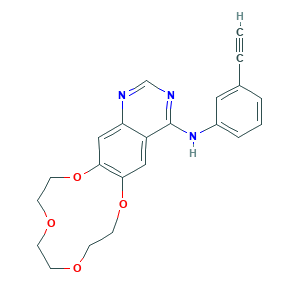
- C22H21N3O4
- Canonical SMILES
- C#CC1=CC(=CC=C1)NC2=NC=NC3=CC4=C(C=C32)OCCOCCOCCO4
- InChI
- 1S/C22H21N3O4/c1-2-16-4-3-5-17(12-16)25-22-18-13-20-21(14-19(18)23-15-24-22)29-11-9-27-7-6-26-8-10-28-20/h1,3-5,12-15H,6-11H2,(H,23,24,25)
- InChIKey
- QQLKULDARVNMAL-UHFFFAOYSA-N
|