| Synonyms |
Ixazomib; Ixazomib (MLN2238); Ixazomib (USAN); Ixazomib Impurity; Ixazomib [INN]; Ixazomib [USAN:INN]; Ixazomib(MLN2238); Ixozamib; MLN 2238; MLN-2238; MLN2238; MLN2238(Ixazomib); SCHEMBL3742758; (R)-(1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutyl)boronic acid; (R)-1-(2-(2,5-dichlorobenzamido)acetamido)-3-methylbutylboronic acid; 1072833-77-2; 71050168A2; C14H19BCl2N2O4; CHEBI:90942; CHEMBL2141296; CTK4A5116; EX-A547; GTPL8450; KS-00000PQX; UNII-71050168A2
|
| Cross-matching ID |
- PubChem CID
- 25183872
- PubChem SID
-
57291607
; 85029113
; 104253504
; 124757626
; 124955652
; 125164430
; 135268985
; 135626848
; 136340279
; 136348738
; 136367593
; 138100925
; 144116241
; 152134539
; 162011779
; 162037798
; 162202726
; 162773434
; 163326535
; 163347633
; 163908014
; 164193936
; 164834143
; 174007116
; 174526063
; 177748936
; 184814067
; 198939582
; 203355961
; 210274689
; 210280323
; 223389604
; 229732516
; 248181513
; 249810660
; 252110098
; 252166660
; 252213184
; 252443130
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D01MML
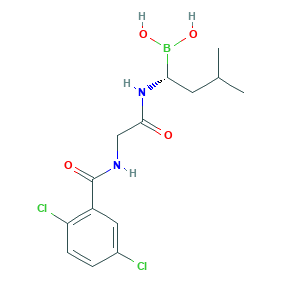
- Formula
- C14H19BCl2N2O4
- Canonical SMILES
- B(C(CC(C)C)NC(=O)CNC(=O)C1=C(C=CC(=C1)Cl)Cl)(O)O
- InChI
- 1S/C14H19BCl2N2O4/c1-8(2)5-12(15(22)23)19-13(20)7-18-14(21)10-6-9(16)3-4-11(10)17/h3-4,6,8,12,22-23H,5,7H2,1-2H3,(H,18,21)(H,19,20)/t12-/m0/s1
- InChIKey
- MXAYKZJJDUDWDS-LBPRGKRZSA-N
|