| General Information of Drug (ID:
DR0921) |
| Drug Name |
Leflunomide
|
| Prodrug Info |
Leflunomide is the prodrug of A77 1726
|
| Synonyms |
Leflunomida; Leflunomida [INN-Spanish]; Leflunomidum; Leflunomidum [INN-Latin]; Lefunomide [Inn-Spanish]; SU 101 (pharmaceutical); SU-101; leflunomide; lefunamide; 5-Methyl-N-(4-(trifluoromethyl)phenyl)-4-isoxazolecarboxamide; Arava (TN); 5-Methylisoxazole-4-carboxylic acid (4-trifluoromethyl)anilide; 5-methyl-N-(4-(trifluoromethyl)phenyl)isoxazole-4-carboxamide; 5-methyl-N-[4-(trifluoromethyl)phenyl]-1,2-oxazole-4-carboxamide; 75706-12-6; Arava; HWA 486; HWA-486; SU101; UNII-G162GK9U4W
|
| Indication |
Rheumatoid arthritis
[ICD11: FA20]
|
Approved
|
[1]
|
| Structure |
|
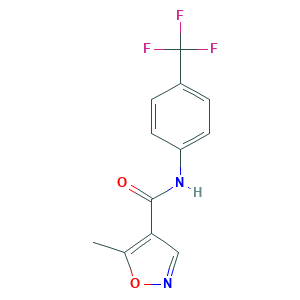 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
270.21 |
Topological Polar Surface Area |
55.1 |
| Heavy Atom Count |
19 |
Rotatable Bond Count |
2 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
6 |
| Cross-matching ID |
- PubChem CID
- 3899
- PubChem SID
-
10107
; 517091
; 604245
; 855764
; 866530
; 6899003
; 7847814
; 7979735
; 8150074
; 8152456
; 10321188
; 11111382
; 11111383
; 11336091
; 11361330
; 11363216
; 11365778
; 11368340
; 11376502
; 11462302
; 11466800
; 11467920
; 11486519
; 11494136
; 11528672
; 11533365
; 12013774
; 15221872
; 17405210
; 24278516
; 26612550
; 26746985
; 26746986
; 26746987
; 29223013
; 46506013
; 47589061
; 47662351
; 47736556
; 47885478
; 48110514
; 48259307
; 48259308
; 48416160
; 49698814
; 49835013
; 50100264
; 50104054
; 50104055
; 50104056
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D08ROP
- Formula
- C12H9F3N2O2
- Canonical SMILES
- CC1=C(C=NO1)C(=O)NC2=CC=C(C=C2)C(F)(F)F
- InChI
- 1S/C12H9F3N2O2/c1-7-10(6-16-19-7)11(18)17-9-4-2-8(3-5-9)12(13,14)15/h2-6H,1H3,(H,17,18)
- InChIKey
- VHOGYURTWQBHIL-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


