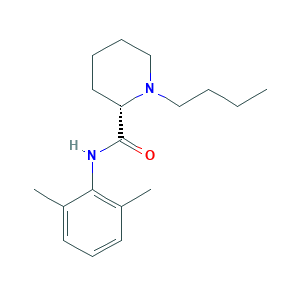
| Synonyms |
Levobupivacaine; Levobupivacaine [INN:BAN]; Levobupivacaine hydrochloride; (2S)-1-butyl-N-(2,6-dimethylphenyl)-2-piperidinecarboxamide; L(-)-Bupivacaine; (2S)-1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide; (S)-1-Butyl-2',6'-pipecoloxylidide; (S)-1-Butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide; (S)-bupivacaine; 2',6'-Pipecoloxylidide, 1-butyl-, L-(-)-; 2-Piperidinecarboxamide, 1-butyl-N-(2,6-dimethylphenyl)-, (2S)-; 27262-47-1; A5H73K9U3W; CHEBI:6149; L-(-)-1-Butyl-2',6'-pipecoloxylidide; UNII-A5H73K9U3W
|
| Cross-matching ID |
- PubChem CID
- 92253
- PubChem SID
-
10089
; 10225294
; 11112642
; 14775644
; 14800000
; 29215441
; 44423110
; 46505295
; 50010834
; 50070564
; 50071325
; 57335205
; 80394341
; 93166629
; 96024806
; 104222110
; 104406939
; 123092444
; 126592946
; 126669543
; 126669544
; 128475942
; 131308447
; 134338472
; 135048182
; 136357477
; 137248692
; 160964338
; 162174730
; 163366975
; 175271332
; 178103786
; 179150260
; 185974045
; 187072685
; 198980870
; 218903197
; 223535869
; 223666603
; 223666604
; 223850360
; 226420984
; 251916836
; 251918075
; 252451169
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D09QUQ
- Formula
- C18H28N2O
- Canonical SMILES
- CCCCN1CCCCC1C(=O)NC2=C(C=CC=C2C)C
- InChI
- 1S/C18H28N2O/c1-4-5-12-20-13-7-6-11-16(20)18(21)19-17-14(2)9-8-10-15(17)3/h8-10,16H,4-7,11-13H2,1-3H3,(H,19,21)/t16-/m0/s1
- InChIKey
- LEBVLXFERQHONN-INIZCTEOSA-N
|