| Synonyms |
Lofexidina; Lofexidina [INN-Spanish]; Lofexidine; Lofexidine [INN:BAN]; Lofexidinum; Lofexidinum [INN-Latin]; Britlofex; 1H-Imidazole, 2-[1-(2,6-dichlorophenoxy)ethyl]-4,5-dihydro-; 2-(1-(2,6-Dichlorophenoxy)ethyl)-4,5-dihydro-1H-imidazole; 2-(alpha-(2,6-Dichlorophenoxy)ethyl)2-imidazoline; 2-Imidazoline 2-(1-(2,6-dichlorophenoxy)ethyl)-; 2-[1-(2,6-dichlorophenoxy)ethyl]-2-imidazoline; 2-[1-(2,6-dichlorophenoxy)ethyl]-4,5-dihydro-1H-imidazole; 31036-80-3; CHEBI:51368; CHEMBL17860
|
| Cross-matching ID |
- PubChem CID
- 30668
- PubChem SID
-
4955007
; 7979787
; 8171702
; 14774610
; 34672885
; 46508453
; 48416180
; 49980645
; 56464309
; 57310968
; 77865548
; 91614206
; 92309039
; 96024831
; 103182297
; 103930569
; 104308830
; 117466455
; 128428177
; 131309952
; 134224363
; 134337392
; 134357768
; 135023284
; 137069359
; 142970890
; 152039684
; 160967834
; 164811810
; 170466275
; 172876170
; 174560267
; 175611898
; 176484253
; 179150300
; 179499877
; 184643900
; 224374099
; 226432808
; 241060023
; 252448593
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0K0MW
- Formula
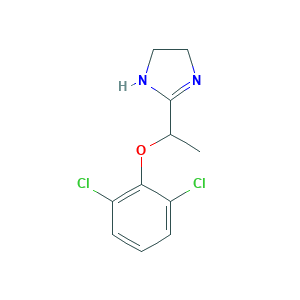
- C11H12Cl2N2O
- Canonical SMILES
- CC(C1=NCCN1)OC2=C(C=CC=C2Cl)Cl
- InChI
- 1S/C11H12Cl2N2O/c1-7(11-14-5-6-15-11)16-10-8(12)3-2-4-9(10)13/h2-4,7H,5-6H2,1H3,(H,14,15)
- InChIKey
- KSMAGQUYOIHWFS-UHFFFAOYSA-N
|