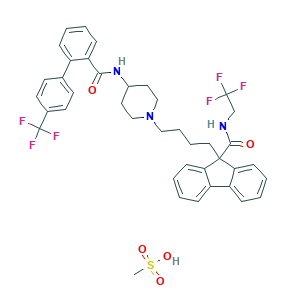
| Synonyms |
Lomitapide (mesylate); Lomitapide mesylate; Lomitapide mesylate (USAN); Lomitapide mesylate [USAN]; X4S83CP54E; lomitapide-mesylate; 202914-84-9; BMS 201038-04; BMS-201038-04; CHEBI:72299; N-(2,2,2-trifluoroethyl)-9-(4-(4-(4'-(trifluoromethyl)biphenyl-2-ylcarboxamido)piperidin-1-yl)butyl)-9H-fluorene-9-carboxamide methanesulfonate; UNII-X4S83CP54E; Juxtapid (TN)
|
| Cross-matching ID |
- PubChem CID
- 11274333
- ChEBI ID
-
- CAS Number
-
- Formula
- C40H41F6N3O5S
- Canonical SMILES
- CS(=O)(=O)O.C1CN(CCC1NC(=O)C2=CC=CC=C2C3=CC=C(C=C3)C(F)(F)F)CCCCC4(C5=CC=CC=C5C6=CC=CC=C64)C(=O)NCC(F)(F)F
- InChI
- 1S/C39H37F6N3O2.CH4O3S/c40-38(41,42)25-46-36(50)37(33-13-5-3-10-30(33)31-11-4-6-14-34(31)37)21-7-8-22-48-23-19-28(20-24-48)47-35(49)32-12-2-1-9-29(32)26-15-17-27(18-16-26)39(43,44)45;1-5(2,3)4/h1-6,9-18,28H,7-8,19-25H2,(H,46,50)(H,47,49);1H3,(H,2,3,4)
- InChIKey
- QKVKOFVWUHNEBX-UHFFFAOYSA-N
|