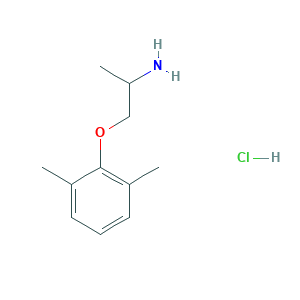
| Synonyms |
Mexiletina; Mexiletina [INN-Spanish]; Mexiletine; Mexiletine [INN:BAN]; Mexiletinum; Mexiletinum [INN-Latin]; Mexilitine; Mexityl; VLPIATFUUWWMKC-UHFFFAOYSA-N; 1-(2',6'-Dimethylphenoxy)-2-aminopropane; 1-(2,6-Dimethylphenoxy)-2-propanamine; 1-(2,6-dimethylphenoxy)propan-2-amine; 1-Methyl-2-(2,6-xylyloxy)ethylamine; 2-(2-aminopropoxy)-1,3-dimethylbenzene; 2-Propanamine, 1-(2,6-dimethylphenoxy)-; 31828-71-4; BRN 2092205; CHEBI:6916; CHEMBL558; EINECS 250-825-7; ETHYLAMINE, 1-METHYL-2-(2,6-XYLYLOXY)-; KO1173; KO 1173; Ko 1173 Cl; Mexiletene hydrochloride; Mexiletine (hydrochloride); Mexiletine HCL; Mexiletine hydrochloride; (S)-1-(2,6-Dimethylphenoxy)propan-2-amine hydrochloride; 1-(2,6-Dimethylphenoxy)-2-propanamine hydrochloride; 1-(2,6-Xylyloxy)-2-aminopropane hydrochloride; 1-(2,6-dimethylphenoxy)propan-2-amine hydrochloride; 2-Propanamine, 1-(2,6-dimethylphenoxy)-, hydrochloride; DSSTox_CID_25783; DSSTox_GSID_45783; DSSTox_RID_81125; EINECS 226-362-1; MFCD00216024; NCGC00094121-01
|