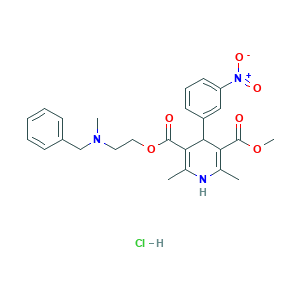
| Synonyms |
Nicardipine LA; Nicardipine [INN:BAN]; Nicardipino; Nicardipino [INN-Spanish]; Nicardipinum; Nicardipinum [INN-Latin]; Angioglebil; Bionicard; Cardene SR; Lincil; NICARDIPINE HYDROCHLORIDE; Nicardil; Nicardipine (Hydrochloride); Nicardipine HCl; Nicodel; Perdipina; Perdipine; RS-69216; RS-69216-XX-07-0; 2-(Benzylmethylamino)ethyl methyl 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)pyridine-3,5-dicarboxylatemonohydrochloride; 54527-84-3; Dafil; Dagan; EINECS 259-198-4; Loxen; MFCD00057327; MLS000069782; Nicardipine hydrochloride [USAN:JAN]; SMR000058487; YC 93; YC-93; ZBBHBTPTTSWHBA-UHFFFAOYSA-N; nicardipine; 3,5-Pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 2-(benzylmethylamino)ethyl methyl ester; 3,5-Pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, methyl 2-(methyl(phenylmethyl)amino)ethyl ester; 55985-32-5; BRN 0504321; C26H29N3O6; CHEBI:7550; DSSTox_CID_3363; EINECS 259-932-3; NCGC00015747-03
|
| Cross-matching ID |
- PubChem CID
- 41114
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0T0KA
- Formula
- C26H30ClN3O6
- Canonical SMILES
- CC1=C(C(C(=C(N1)C)C(=O)OCCN(C)CC2=CC=CC=C2)C3=CC(=CC=C3)[N+](=O)[O-])C(=O)OC.Cl
- InChI
- 1S/C26H29N3O6.ClH/c1-17-22(25(30)34-4)24(20-11-8-12-21(15-20)29(32)33)23(18(2)27-17)26(31)35-14-13-28(3)16-19-9-6-5-7-10-19;/h5-12,15,24,27H,13-14,16H2,1-4H3;1H
- InChIKey
- AIKVCUNQWYTVTO-UHFFFAOYSA-N
|