| General Information of Drug (ID:
DR1146) |
| Drug Name |
Niclosamide
|
| Synonyms |
Nasemo; Niclocide; Phenasal; Radeverm; Radewerm; Sagimid; Sulqui; Tredemine; Vermitid; Yomesan; Zestocarp; niclosamide; nicolsamide; Atenase; Bayluscid; Cestocid; Devermin; Devermine; Dichlosale; Fedal-Telmin; Fenasal; Helmiantin; Iomesan; Iomezan; Lintex; Mansonil; 2',5-Dichloro-4'-nitrosalicylanilide; 5-Chloro-2'-chloro-4'-nitrosalicylanilide; 5-Chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide; 50-65-7; BAY 2353; Bayer 2353; Bayer 73; Benzamide, 5-chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxy-; Chemagro 2353; HL 2447; Mato
|
| Indication |
Tapeworm infestation
[ICD11: 1F7Y]
|
Approved
|
[1]
|
| Structure |
|
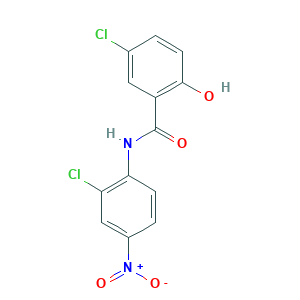 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
327.12 |
Topological Polar Surface Area |
95.2 |
| Heavy Atom Count |
21 |
Rotatable Bond Count |
2 |
| Hydrogen Bond Donor Count |
2 |
Hydrogen Bond Acceptor Count |
4 |
| Cross-matching ID |
- PubChem CID
- 4477
- PubChem SID
-
446537
; 635963
; 5010826
; 5010827
; 7847502
; 7980120
; 8149966
; 8152760
; 10321405
; 10852038
; 11111533
; 11111534
; 11335884
; 11361123
; 11363133
; 11365695
; 11368257
; 11371653
; 11373910
; 11376419
; 11462095
; 11466068
; 11467188
; 11484961
; 11485796
; 11488999
; 11490483
; 11492011
; 11494053
; 12159141
; 14924163
; 17405839
; 24278585
; 24862257
; 26612455
; 26680237
; 26747596
; 26753613
; 29223572
; 46511793
; 47242136
; 47365202
; 47662299
; 47810766
; 47885430
; 47959760
; 48035132
; 48035133
; 48259243
; 48334504
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0J9ZR
- Formula
- C13H8Cl2N2O4
- Canonical SMILES
- C1=CC(=C(C=C1[N+](=O)[O-])Cl)NC(=O)C2=C(C=CC(=C2)Cl)O
- InChI
- 1S/C13H8Cl2N2O4/c14-7-1-4-12(18)9(5-7)13(19)16-11-3-2-8(17(20)21)6-10(11)15/h1-6,18H,(H,16,19)
- InChIKey
- RJMUSRYZPJIFPJ-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


