| General Information of Drug (ID:
DR1160) |
| Drug Name |
Nimesulide
|
| Synonyms |
Nimesulida [INN-Spanish]; Nimesulidum [INN-Latin]; Nisulid; Sulidene; V4TKW1454M; nimesulide; 4'-Nitro-2'-phenoxymethanesulfonanilide; Flogovital; Mesulid; 4'-Nitro-2'-phenoxymethansulfonanilid; 4-NITRO-2-PHENOXYMETHANESULFONANILIDE; 4-Nitro-2-phenoxy-methanesulfonanilide; 51803-78-2; BRN 2421175; CHEBI:44445; CHEMBL56367; EINECS 257-431-4; MLS000069680; Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-; Methanesulfonanilide, 4'-nitro-2'-phenoxy-; N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide; Nimed; R 805; R-805; UNII-V4TKW1454M
|
| Indication |
Anaesthesia
[ICD11: 8E22]
|
Phase 4
|
[1]
|
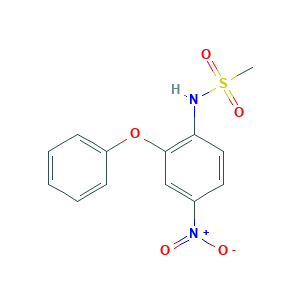
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
308.31 |
Topological Polar Surface Area |
110 |
| Heavy Atom Count |
21 |
Rotatable Bond Count |
4 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
6 |
| Cross-matching ID |
- PubChem CID
- 4495
- PubChem SID
-
855635
; 5912186
; 6436673
; 7404099
; 7848112
; 7889413
; 7980127
; 8149946
; 8152768
; 10321585
; 11111520
; 11111521
; 11121727
; 11122207
; 11335868
; 11361107
; 11362857
; 11364471
; 11365419
; 11367033
; 11367981
; 11369595
; 11370925
; 11370926
; 11372711
; 11373582
; 11373834
; 11376143
; 11377757
; 11462079
; 11466222
; 11467342
; 11485118
; 11485804
; 11489357
; 11491391
; 11491993
; 11495391
; 12012650
; 14752054
; 17405416
; 24277576
; 24278583
; 26612437
; 26680139
; 26697157
; 26746940
; 26746941
; 26751468
; 29215000
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0C0JT
- Formula
- C13H12N2O5S
- Canonical SMILES
- CS(=O)(=O)NC1=C(C=C(C=C1)[N+](=O)[O-])OC2=CC=CC=C2
- InChI
- 1S/C13H12N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h2-9,14H,1H3
- InChIKey
- HYWYRSMBCFDLJT-UHFFFAOYSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


