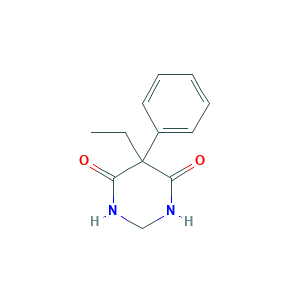
| Cross-matching ID |
- PubChem CID
- 4909
- PubChem SID
-
9575
; 96478
; 855864
; 5227599
; 7847540
; 7980382
; 8149495
; 8153020
; 10506074
; 11111659
; 11112270
; 11113350
; 11335499
; 11360738
; 11363726
; 11366288
; 11368850
; 11371825
; 11374108
; 11377012
; 11461710
; 11466961
; 11468081
; 11485061
; 11486771
; 11489109
; 11490369
; 11492300
; 11494646
; 15121366
; 17389534
; 17405579
; 24277732
; 26611888
; 26679596
; 26747018
; 26747019
; 26751542
; 29223987
; 46507775
; 47291047
; 47365104
; 47440159
; 47440160
; 47440161
; 47440162
; 47588907
; 47810657
; 48184911
; 48184912
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0U5RT
- Formula
- C12H14N2O2
- Canonical SMILES
- CCC1(C(=O)NCNC1=O)C2=CC=CC=C2
- InChI
- 1S/C12H14N2O2/c1-2-12(9-6-4-3-5-7-9)10(15)13-8-14-11(12)16/h3-7H,2,8H2,1H3,(H,13,15)(H,14,16)
- InChIKey
- DQMZLTXERSFNPB-UHFFFAOYSA-N
|