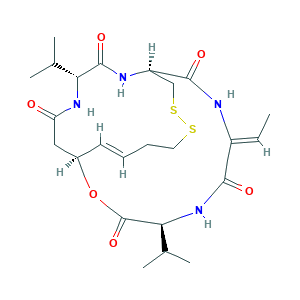
| Synonyms |
Romidepsin; FK 228; FK-228; FK228; FR 901228; FR-901228; FR901228; NSC 630176; NSC-630176; NSC630176; NSC754143; (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone; (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetrazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone; 128517-07-7; C24H36N4O6S2; CHEBI:61080; CHEMBL343448; Antibiotic FR 901228; Chromadax; Depsipeptide; Istodax; UNII-CX3T89XQBK
|
| Cross-matching ID |
- PubChem CID
- 5352062
- PubChem SID
-
494874
; 8139716
; 11650994
; 12014448
; 14763476
; 14763478
; 35018633
; 46519185
; 47208288
; 47721651
; 47944836
; 48394948
; 50062237
; 50260193
; 53788098
; 57361456
; 87226502
; 103393180
; 104222481
; 113905898
; 127325932
; 127325933
; 131408687
; 134338801
; 134338973
; 134340279
; 137156420
; 139110210
; 152159610
; 163620827
; 163686155
; 164233439
; 175266198
; 176484649
; 178103585
; 184816960
; 198992824
; 224274473
; 226087966
; 226972555
; 251894761
; 252430794
; 252450287
; 252473284
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0L7LC
- Formula
- C24H36N4O6S2
- Canonical SMILES
- CC=C1C(=O)NC(C(=O)OC2CC(=O)NC(C(=O)NC(CSSCCC=C2)C(=O)N1)C(C)C)C(C)C
- InChI
- 1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17-,19-,20+/m1/s1
- InChIKey
- OHRURASPPZQGQM-GCCNXGTGSA-N
|