| Synonyms |
Trimanyl; Trimethioprim; Trimethoprime; Trimethoprimum; Trimetoprim; Trimetoprima; Trimopan; Trimpex; Triprim; Trisulcom; Trisulfam; Uretrim; Apo-Sulfatrim; Bacticel; Bactramin; Chemotrin; Cotrimel; Espectrin; Fermagex; Lagatrim; Monoprim; Monotrim; Monotrimin; NIH 204; Novotrimel; Pancidim; Proloprim; Sinotrim; Sugaprim; Sulfamar; Sulfamethoprim; Sulfoxaprim; Syraprim; Urobactrim; Wellcoprim; Wellcoprin; Zamboprim; trimethoprim; 2,4-Diamino-5-(3,4,5-trimethoxybenzyl)pyrimidine; 5-(3,4,5-trimethoxybenzyl)pyrimidine-2,4-diamine; 738-70-5; Abaprim
|
| Cross-matching ID |
- PubChem CID
- 5578
- PubChem SID
-
5066
; 406777
; 604810
; 837167
; 857743
; 3146445
; 4752045
; 7847213
; 7890832
; 7980835
; 8027414
; 8149571
; 8153433
; 10321479
; 10534335
; 11111923
; 11111924
; 11113621
; 11335764
; 11361003
; 11363061
; 11365623
; 11368185
; 11371565
; 11374002
; 11376347
; 11461975
; 11466236
; 11467356
; 11484610
; 11485852
; 11488753
; 11490459
; 11492187
; 11493981
; 14824824
; 17389524
; 17405825
; 22391545
; 24278201
; 24870616
; 24889615
; 25623645
; 26611964
; 26680042
; 26697109
; 26704777
; 26747124
; 26747125
; 26751820
- ChEBI ID
-
- CAS Number
-
- TTD Drug ID
- D0AO5H
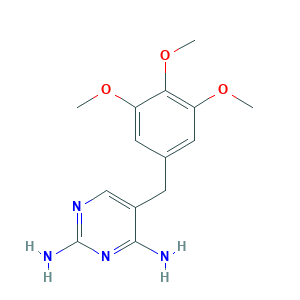
- Formula
- C14H18N4O3
- Canonical SMILES
- COC1=CC(=CC(=C1OC)OC)CC2=CN=C(N=C2N)N
- InChI
- 1S/C14H18N4O3/c1-19-10-5-8(6-11(20-2)12(10)21-3)4-9-7-17-14(16)18-13(9)15/h5-7H,4H2,1-3H3,(H4,15,16,17,18)
- InChIKey
- IEDVJHCEMCRBQM-UHFFFAOYSA-N
|