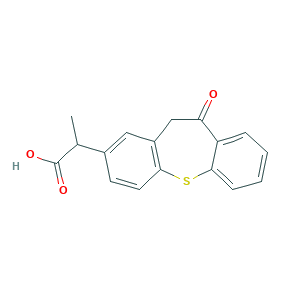
| Synonyms |
Zaltoprofen (unspecified); Zaltoprofene; Zaltoprofeno; Zaltoprofenum; CN 100; CN-100; Soleton; zaltoprofen; (+-)-2-(10,11-Dihydro-10-oxodibenzo(b,f)thiepin-2-yl)propionic acid; 10,11-Dihydro-alpha-methyl-10-oxodibenzo(b,f)thiepin-2-acetic acid; 2-(10,11-Dihydro-10-oxodibenzo[b,f]thiepin-2-yl)propionic Acid; 2-(10-oxo-10,11-dihydrodibenzo[b,f]thiepin-2-yl)propanoic acid; 2-(6-oxo-5H-benzo[b][1]benzothiepin-3-yl)propanoic acid; 74711-43-6; 89482-00-8; AK-77321; C17H14O3S; EINECS 277-973-5; NCGC00183878-01; Peon
|
| Cross-matching ID |
- PubChem CID
- 5720
- ChEBI ID
-
- CAS Number
-
- Formula
- C17H14O3S
- Canonical SMILES
- CC(C1=CC2=C(C=C1)SC3=CC=CC=C3C(=O)C2)C(=O)O
- InChI
- 1S/C17H14O3S/c1-10(17(19)20)11-6-7-15-12(8-11)9-14(18)13-4-2-3-5-16(13)21-15/h2-8,10H,9H2,1H3,(H,19,20)
- InChIKey
- MUXFZBHBYYYLTH-UHFFFAOYSA-N
|