| General Information of Drug (ID:
DR1775) |
| Drug Name |
TRV-130
|
| Synonyms |
Oliceridine; Oliceridine (USAN/INN); Oliceridine [USAN]; MCN858TCP0; Olinvo; TRV 130; TRV-130; TRV130; TRV130A; ZINC96940334; 1401028-24-7; 6-Oxaspiro(4.5)decane-9-ethanamine, N-((3-methoxy-2-thienyl)methyl)-9-(2-pyridinyl)-, (9R)-; 6-Oxaspiro[4.5]decane-9-ethanamine, N-[(3-methoxy-2-thienyl)methyl]-9-(2-pyridinyl)-, (9R)-; BCP14196; CHEMBL2443262; EX-A2170; GTPL7334; SCHEMBL12542370; UNII-MCN858TCP0; [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro[4.5]decan-9-yl]ethyl})amine
|
| Indication |
Hypothyroidism
[ICD11: 5A00]
|
Phase 1/2
|
[1]
|
| Structure |
|
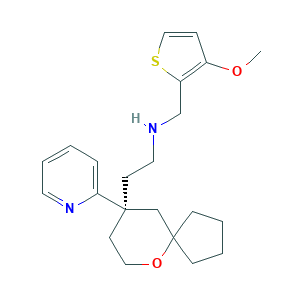 |
|
3D MOL
|
2D MOL
|
| Pharmaceutical Properties |
Molecular Weight |
386.6 |
Topological Polar Surface Area |
71.6 |
| Heavy Atom Count |
27 |
Rotatable Bond Count |
7 |
| Hydrogen Bond Donor Count |
1 |
Hydrogen Bond Acceptor Count |
5 |
| Cross-matching ID |
- PubChem CID
- 66553195
- PubChem SID
-
152159759
; 174525413
; 178103906
; 237602152
; 252163523
- CAS Number
-
- TTD Drug ID
- D06ENM
- Formula
- C22H30N2O2S
- Canonical SMILES
- COC1=C(SC=C1)CNCCC2(CCOC3(C2)CCCC3)C4=CC=CC=N4
- InChI
- 1S/C22H30N2O2S/c1-25-18-7-15-27-19(18)16-23-13-10-21(20-6-2-5-12-24-20)11-14-26-22(17-21)8-3-4-9-22/h2,5-7,12,15,23H,3-4,8-11,13-14,16-17H2,1H3/t21-/m1/s1
- InChIKey
- DMNOVGJWPASQDL-OAQYLSRUSA-N
|
|
|
|
|
|
|
|
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.


