Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1825) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
UK-453,061
|
|||||
| Synonyms |
Lersivirine; Lersivirine [USAN:INN]; R3ZGC15A9A; UK 453,061; UK-453,061; UK-453061; 2won; 3-CYANO-5-[[3,5-DIETHYL-1-(2-HYDROXYETHYL)-1H-PYRAZOL-4-YL]OXY]BENZONITRILE; 473921-12-9; 5-((3,5-Diethyl-1-(2-hydroxyethyl)-1H-pyrazol-4-yl)oxy)benzene-1,3-dicarbonitrile; 5-((3,5-diethyl-1-(2-hydroxyethyl)-1H-pyrazol-4-yl)oxy)isophthalonitrile; 5-[3,5-diethyl-1-(2-hydroxyethyl)pyrazol-4-yl]oxybenzene-1,3-dicarbonitrile; 5-{[3,5-Diethyl-1-(2-Hydroxyethyl)-1h-Pyrazol-4-Yl]oxy}benzene-1,3-Dicarbonitrile; UNII-R3ZGC15A9A; ZZE
|
|||||
| Indication | Human immunodeficiency virus infection [ICD11: 1C60] | Phase 2 | [1] | |||
| Structure |
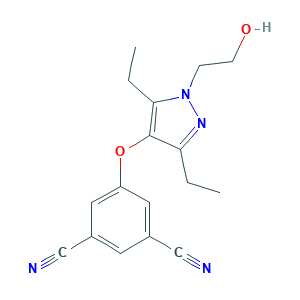 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 310.35 | Topological Polar Surface Area | 94.9 | ||
| Heavy Atom Count | 23 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 5 | |||
| Cross-matching ID | ||||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||
| References | ||||||
|---|---|---|---|---|---|---|
| 1 | ClinicalTrials.gov (NCT01254656) A Long Term Safety Study Of Lersivirine For The Treatment Of HIV-1 Infection In Subjects Who Have Completed Treatment With Lersivirine In Studies A5271015 And A5271022. | |||||
| 2 | Excretion and metabolism of lersivirine (5-{[3,5-diethyl-1-(2-hydroxyethyl)(3,5-14C2)-1H-pyrazol-4-yl]oxy}benzene-1,3-dicarbonitrile), a next-generation non-nucleoside reverse transcriptase inhibitor, after administration of [14C]Lersivirine to healthy volunteers. Drug Metab Dispos. 2010 May;38(5):789-800. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

