Details of the Drug Metabolized by Drug-Metabolizing Enzyme (DME)
| General Information of Drug (ID: DR1849) | ||||||
|---|---|---|---|---|---|---|
| Drug Name |
CP-122721
|
|||||
| Synonyms |
CP-122721; R7OYP6N58F; SCHEMBL156646; Tox21_300205; ZINC22441997; (2S,3S)-N-(2-methoxy-5-(trifluoromethoxy)benzyl)-2-phenylpiperidin-3-amine; BDBM50067935; CAS-145742-28-5; CHEMBL1917847; CHEMBL319118; DB05421; DSSTox_CID_27251; DSSTox_GSID_47251; DSSTox_RID_82210; DTXSID9047251; NCGC00247927-01; NCGC00254228-01; UNII-R7OYP6N58F
|
|||||
| Indication | Depression [ICD11: 6A71] | Phase 2 | [1] | |||
| Structure |
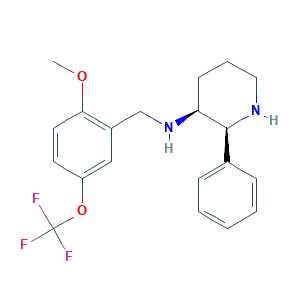 |
|||||
| 3D MOL | 2D MOL | |||||
| Pharmaceutical Properties | Molecular Weight | 380.4 | Topological Polar Surface Area | 42.5 | ||
| Heavy Atom Count | 27 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 7 | |||
| Cross-matching ID |
|
|||||
| The Metabolic Roadmap of This Drug | |||||
|---|---|---|---|---|---|
| The Full List of Drug Metabolites (DM) of This Drug | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Full List of Metabolic Reaction (MR) of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug-Metabolizing Enzyme(s) (DME) Metabolizing This Drug | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.

